Printed from acutecaretesting.org
October 2004
Jaundice in the newborn infant
Introduction
About half of all newborn infants born at term (gestational age > 37 weeks) develop jaundice during their first days of life, and the lower the gestational age the more frequent the jaundice in the infants.Bilirubin metabolism (Fig. 1)
From RES bilirubin is released in plasma where it binds very tightly to albumin and is transported to the liver, where the liver cells take it up. Here and with the aid of ligandin, bilirubin is transported to the endoplasmatic reticulum, where it is conjugated to bilirubin diglucuronide by binding to glucuronic acid with the aid of uridine diphosphoglucuronyl transferase. This makes bilirubin more water-soluble and it can be excreted with the bile.
In the intestinal lumen during the first week of life, β-glucuronidase may split off glucuronic acid after which bilirubin may be reabsorbed from the intestine. Thus, there is an enterohepatic recirculation of unconjugated bilirubin.
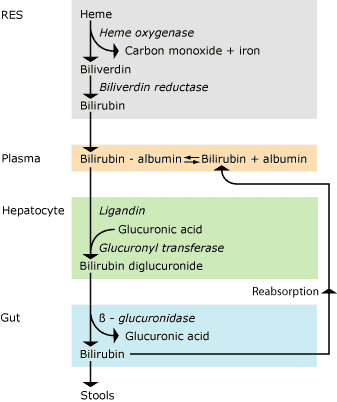
FIG. 1. Bilirubin metabolism. RES: Reticuloendothelial system
Hyperbilirubinemia in the newborn
Newborns develop hyperbilirubinemia because of:- a large bilirubin production, twice as large per kg bodyweight as in adults, caused by a high hematocrit and a short erythrocyte lifetime (90 days in term infants)
- a low excretion of bilirubin, as the hepatic processes are immature
- the enterohepatic recirculation
At birth, the newborn is not icteric, as bilirubin can pass the placenta and be excreted by the mother. At birth, the newborn’s plasma bilirubin concentration is slightly higher than that of the mother, 15-40 µmol/L. After birth, the infant’s plasma bilirubin concentration increases. If it exceeds 60-80 µmol/L, a yellow coloring of the skin will appear [1].
It starts in the face and spreads from there down across the body and extremities to the palms of the hands and the soles of the feet [2]. In term newborns the hyperbilirubinemia most often culminates on day 3-5 of life, in preterm newborns most often on day 5-7 of life. The reason for this is that the liver function matures and the enterohepatic recirculation decreases.
The latter because the gut is colonized by bacteria that can transform bilirubin into stercobilin, which is then excreted with the stools. The average maximum plasma bilirubin concentration in term newborns is approx. 90-100 µmol/L. In uncomplicated hyperbilirubinemia almost all bilirubin in plasma is unconjugated and the conjugated fraction is negligible [3].
The severity of the jaundice depends on:
- Gestational age. The lower the gestational age, the more immature the liver and thus the higher the plasma bilirubin concentration.
- Gender. Boys have a higher bilirubin concentration than girls [4].
- Family factors. If an infant has had severe jaundice, there is an increased risk that its siblings will also get it [5].
- Race. In average, the Mongoloid race has a higher plasma bilirubin concentration than the Caucasian race, which again has a higher concentration than the Negroid race [4].
- Nutrition. Infants feeding on breast milk have a higher bilirubin concentration than infants feeding on formula, so-called breast milk jaundice [6].
- Time of the first meal and frequency of meals. The earlier the infant has its first meal and the more meals it has, the lower the plasma bilirubin concentration, as the enterohepatic recirculation is thereby reduced due to a reduced gut transit time [7].
A contributing factor to an increased plasma bilirubin concentration can be polycytemia, cephalhematoma and suggillations. Additional factors can be hemolysis by isoimmunization (Rhesus and ABO blood type immunization) or spherocytosis, erythrocyte glucose-6-phosphate dehydrogenase deficiency, galactosemia, sepsis, intestinal obstruction, hepatitis, etc.
Only unconjugated bilirubin is toxic. Its toxic effect on cells is still not quite clear. In high plasma concentrations, bilirubin as acid can precipitate in phospholipid membranes. The toxicity of bilirubin is therefore considered to be pH-dependent [8].
When plasma bilirubin concentration becomes very high, infants can have signs and symptoms of central nervous system involvement, acute bilirubin encephalopathy. In phase 1, the infants may display the following clinical symptoms: Lethargy, poor sucking, hypotonia, stupor and a weak Moro reflex. A few cohort studies [9,10] have found an association between hyperbilirubinemia and subtle long-term effects in term infants, but in most studies such a relationship has not been observed.
In phase 2 (classic kernicterus) the infants may have the following symptoms: Hypertonicity, opistotonus, pronation spasms of the arms, seizures, apnea, fever and a high-pitched cry (Fig. 2.). These infants almost always suffer permanent brain damage such as reduced hearing, dystonic or athethoid cerebral palsy, retarded psychomotor development and paralysis of upward gaze.
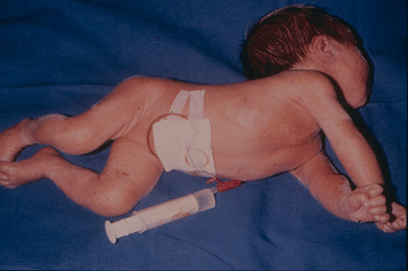
FIG. 2. The infant was born near term and had extreme hyperbilirubinemia due to Rhesus blood type immunization. He developed classic kernicterus with opistotonus, hypertonicity, pronation spasms of the upper extremities, seizures and fever.
By kernicterus there is a preferential disposition of bilirubin in the globus pallidus, subthalamic nuclei, hippocampus, putamen, thalamus and cranial nerve nuclei, especially 3, 4 and 6 [11]. By magnetic resonance imaging (MRI), abnormalities are seen in the globus pallidus and subthalamic nuclei [12].
In recent years, the industrialized world has seen an increasing number of term or near-term infants with classic kernicterus [13,14].
The reasons for this may be:
- The infants are discharged earlier from the maternity ward, so that the jaundice culminates after discharge from the ward.
- The risk of kernicterus in term or near-term infants has been underestimated.
- Only very few doctors have seen infants suffering from classic kernicterus.
- The number of immigrants has increased. It can be clinically difficult to assess the intensity of jaundice with dark skin.
- More mothers wish to breastfeed their newborn babies, leading to an increased frequency of hyperbilirubinemia, so-called breastfeeding-associated jaundice [6]. This is partly because feeding on breast milk, as mentioned, leads to high plasma bilirubin concentration, partly because breastfed infants often receive less nutrition than formula-fed infants.
Monitoring
The initial screening for jaundice is done
visually, assessing partly its intensity and partly how far it
has progressed. On the basis of this it is decided if it is
necessary to measure the transcutaneous bilirubin concentration
with a bilirubinometer. This measurement is made either on the
forehead or on the chest. From the instrument a bundle of light
is transmitted to the skin and the reflected light is analyzed
[15].
If the transcutaneous bilirubin concentration exceeds a certain limit, plasma bilirubin concentration is determined [16]. Measurement of transcutaneous bilirubin concentration is mainly employed on maternity wards in healthy term or near-term infants (gestational age 35-36 weeks), but it can with advantage also be employed on the neonatal intensive care units in infants of a gestational age > 32 weeks [16].
Due to the increased number of infants with classic kernicterus and the difficulty of assessing the intensity of jaundice in infants with dark skin, a screening of term and near-term infants has been proposed by measurement of the transcutaneous bilirubin concentration [15], the end-expiratory carbon monoxide or a combination of both [17]. The 2004 guidelines from AAP (American Academy of Pediatrics) advise a systematic assessment before discharge for the risk of hyperbilirubinemia. This may be done by measuring the bilirubin level using concentration of plasma total bilirubin or transcutaneous bilirubin and/or assessing clinical risk factors [18].
On a routine basis plasma total bilirubin concentration is determined, and it is regarded as identical to the concentration of unconjugated bilirubin as the concentration of conjugated bilirubin in newborns without liver disease is, as mentioned, negligible [3]. Upon suspected liver disease, fractional bilirubin is determined, i.e. the concentration of unconjugated as well as conjugated bilirubin.
Determination of free bilirubin in plasma is primarily used for research purposes. Development of jaundice within the first 24 hours of life always requires determination of plasma bilirubin.
Treatment
Phototherapy
Phototherapy is the primary treatment of choice.
Irradiation of the skin with blue or blue-green light
transforms bilirubin into photobilirubins, which are isomer
compounds of bilirubin. Whereas bilirubin is lipid-soluble and
very hard to dissolve in water, photobilirubins are more polar
and dissolve easily in water.
They can be excreted through the liver to the bile without conjugation. Light affects the skin to a depth of 1-2 mm [19] and the yellow coloring of the skin disappears in the irradiated areas. The infants’ eyes must be covered during the treatment to avoid the risk of injuring the retina. Phototherapy has no side effects of significance, but
- the infants often suffer from secretory diarrhea [20]
- there is an increased insensible water loss from the skin. Therefore preterm infants need extra fluid, which is especially important in extreme preterm infants
- an exanthem may be observed
- when exposing infants with liver disease to light, a gray-brown discoloring of skin, plasma and urine can be observed, a condition known as the bronze- baby syndrome. The pigments are created when exposing bilirubin and copper porphyrins to light [21]. The condition is presumably of no importance [22].
Exchange transfusion
By exchange transfusion the infant’s blood is exchanged, often
through the umbilical vein, with a volume twice the infant’s
blood volume, so that a major part of bilirubin is removed.
This procedure entails a significant morbidity in the form of
necrotizing enterocolitis, circulatory collapse and sepsis,
even mortality.
Tin-mesoporphyrin injection [23]
This is a very promising treatment, which has not come into
clinical use yet. Tin-mesoporphyrin is a heme analog that
competes with heme for the binding sites of heme oxygenase.
This reduces the breaking down of heme and thus the production
of bilirubin, reducing the need for phototherapy
considerably. It can be used prophylactically and in
advanced hyperbilirubinemia. The treatment seems to have no
side effects of significance.
Prevention of acute bilirubin encephalopathy
The following could presumably assist in reducing
the incidence of severe hyperbilirubinemia:
- A reconsidered view of the risk of acute bilirubin encephalopathy with very high plasma bilirubin concentrations, as it seems to be greater than generally thought.
- Ensuring that all mothers after birth receive both verbal and written information on jaundice in the newborn infant.
- Be more liberal in giving infant formula supplements in situations where breastfeeding is difficult to establish.
- Screening of infants of non-Caucasian origin for the risk of developing a high plasma bilirubin concentration might be reasonable.
- When authorized guidelines for phototherapy and exchange transfusions exist, they should be followed.
References+ View more
- Riskin A, Abend-Weinger M, Bader D. How accurate are neonatologists in identifying clinical jaundice in newborns? Clin Pediatr 2003; 42,2: 153-58.
- Kramer LI. Advancement of dermal icterus in the jaundiced newborn. Am J Dis Child 1969; 118: 454-58.
- Brodersen R, Jacobsen J. Serum bilirubin diglucoronide in the human adult and the newborn child. In: Bilirubin metabolism. Oxford: Blackwell scientific press, 1967; 111-15.
- Linn S, Schoenbaum SC, Monson RR, Rosner B, Stubbefield PG, Ryan KJ. Epidemiology of neonatal hyperbilirubinemia. Pediatrics 1985; 75: 770-74.
- Nielsen HE, Haase P, Blaabjerg J, Stryhn H, Hilden J. Risk factors and sib correlation in physiological neonatal jaundice. Acta Paediatrica Scand 1987; 76: 504-11.
- Auerbach KG, Gartner LM. Breast feeding and human milk: their association with jaundice in the neonate. Clin Perinatol 1987; 14: 89-107.
- DeCarvalho M. Klaus MH, Merkatz RB. Frequency of breast-feeding and serum bilirubin concentration. Am J Dis Child 1982; 136: 737-38.
- Burgess GH, Oh W, Bratlid D, Brubakk AM, Cashore WJ, Stonestreet BS. Effects of acidosis on bilirubin deposition in rat brain. Pediatr Res 1985; 19: 691-96.
- Soorani-Lunsing I, Woltil H, Hadders-Algra M. Are moderate degrees of hyperbilirubinemia in healthy term neonates safe for the brain? Pediatr Res 2001; 50: 701-05.
- Seidman DS, Paz I, Stevenson DK, Laor A, DanonYL, Gale R. Neonatal hyperbilirubinemia and physical and cognitive performance at 17 years of age. Pediatrics 1991; 88: 828-33.
- Turkel SB, Miller CA, Guttenberg ME, Moynes DR, Hodgman JE. A clinical pathological reappraisal of kernicterus. Pediatrics 1982; 69: 267-72.
- Shapiro SM, O’Tuama LA, Stringer WA, Baron MS. Magnetic resonance imaging (MRI) findings in 26 children with clinical kernicterus. Pediatr Res 2004; 55: 432 A.
- Brown A, Johnson L. Loss of concern about jaundice and the reemergency of kernicterus in full-term infants in an era of managed care. In: Year book of neonatal and perinatal medicine. St Louis, MO: Mosby Year Book, 1996: 17-28.
- Ebbesen F. Recurrence of kernicterus in term and near-term infants in Denmark. Acta Paediatrica 2000; 89:1213-17.
- Bhutani VK, Gourley GR, Adler S, Kreamer B, Dahlin C, Johnson LH. Noninvasive measurement of total bilirubin in a multiracial predischarge population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000; 106:e17.
- Ebbesen F, Rasmussen LM, Wimberley PD . A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatrica 2002; 91: 203-11.
- Stevenson D, Fanaroff A, Maisels J, Young B, Wong R, Vreman H et al. Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics 2001; 108: 31-9.
- Maisels MJ, Baltz RD, Bhutani VK et al. Pediatrics 2004; 114: 297-316.
- Vogl T. Phototherapy of neonatal hyperbilirubinemia: bilirubin in unexposed areas of the skin. Pediatrics 1972; 85: 707-10.
- de Curtis M, Saitta F, Matteoli M, Paludetto R, Ciccimarra F, Guandalini S. Evidence for secretory type of diarrhoea in infants treated by phototherapy. Lancet 1982; i: 909.
- Rubaltelli FF, Da Riol R, D’Amore ESG, Jori G. The bronze baby syndrome: evidence of increased tissue concentration of copper porphyrins. Acta Paediatrica 1996; 85: 381-84.
- Tan KL, Jacob E. The bronze baby syndrome. Acta Paediatrica Scand 1982; 71: 409-14.
- Kappas A. A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics 2004; 113: 119-23.
References
- Riskin A, Abend-Weinger M, Bader D. How accurate are neonatologists in identifying clinical jaundice in newborns? Clin Pediatr 2003; 42,2: 153-58.
- Kramer LI. Advancement of dermal icterus in the jaundiced newborn. Am J Dis Child 1969; 118: 454-58.
- Brodersen R, Jacobsen J. Serum bilirubin diglucoronide in the human adult and the newborn child. In: Bilirubin metabolism. Oxford: Blackwell scientific press, 1967; 111-15.
- Linn S, Schoenbaum SC, Monson RR, Rosner B, Stubbefield PG, Ryan KJ. Epidemiology of neonatal hyperbilirubinemia. Pediatrics 1985; 75: 770-74.
- Nielsen HE, Haase P, Blaabjerg J, Stryhn H, Hilden J. Risk factors and sib correlation in physiological neonatal jaundice. Acta Paediatrica Scand 1987; 76: 504-11.
- Auerbach KG, Gartner LM. Breast feeding and human milk: their association with jaundice in the neonate. Clin Perinatol 1987; 14: 89-107.
- DeCarvalho M. Klaus MH, Merkatz RB. Frequency of breast-feeding and serum bilirubin concentration. Am J Dis Child 1982; 136: 737-38.
- Burgess GH, Oh W, Bratlid D, Brubakk AM, Cashore WJ, Stonestreet BS. Effects of acidosis on bilirubin deposition in rat brain. Pediatr Res 1985; 19: 691-96.
- Soorani-Lunsing I, Woltil H, Hadders-Algra M. Are moderate degrees of hyperbilirubinemia in healthy term neonates safe for the brain? Pediatr Res 2001; 50: 701-05.
- Seidman DS, Paz I, Stevenson DK, Laor A, DanonYL, Gale R. Neonatal hyperbilirubinemia and physical and cognitive performance at 17 years of age. Pediatrics 1991; 88: 828-33.
- Turkel SB, Miller CA, Guttenberg ME, Moynes DR, Hodgman JE. A clinical pathological reappraisal of kernicterus. Pediatrics 1982; 69: 267-72.
- Shapiro SM, O’Tuama LA, Stringer WA, Baron MS. Magnetic resonance imaging (MRI) findings in 26 children with clinical kernicterus. Pediatr Res 2004; 55: 432 A.
- Brown A, Johnson L. Loss of concern about jaundice and the reemergency of kernicterus in full-term infants in an era of managed care. In: Year book of neonatal and perinatal medicine. St Louis, MO: Mosby Year Book, 1996: 17-28.
- Ebbesen F. Recurrence of kernicterus in term and near-term infants in Denmark. Acta Paediatrica 2000; 89:1213-17.
- Bhutani VK, Gourley GR, Adler S, Kreamer B, Dahlin C, Johnson LH. Noninvasive measurement of total bilirubin in a multiracial predischarge population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000; 106:e17.
- Ebbesen F, Rasmussen LM, Wimberley PD . A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatrica 2002; 91: 203-11.
- Stevenson D, Fanaroff A, Maisels J, Young B, Wong R, Vreman H et al. Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics 2001; 108: 31-9.
- Maisels MJ, Baltz RD, Bhutani VK et al. Pediatrics 2004; 114: 297-316.
- Vogl T. Phototherapy of neonatal hyperbilirubinemia: bilirubin in unexposed areas of the skin. Pediatrics 1972; 85: 707-10.
- de Curtis M, Saitta F, Matteoli M, Paludetto R, Ciccimarra F, Guandalini S. Evidence for secretory type of diarrhoea in infants treated by phototherapy. Lancet 1982; i: 909.
- Rubaltelli FF, Da Riol R, D’Amore ESG, Jori G. The bronze baby syndrome: evidence of increased tissue concentration of copper porphyrins. Acta Paediatrica 1996; 85: 381-84.
- Tan KL, Jacob E. The bronze baby syndrome. Acta Paediatrica Scand 1982; 71: 409-14.
- Kappas A. A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics 2004; 113: 119-23.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








