Printed from acutecaretesting.org
May 2014
Laboratory use of umbilical cord blood for critically ill infants
Premature and critically ill infants in the NICU are frequently transfused
Rates of transfusion are higher in neonates than any other pediatric population. The smallest neonates have an exceedingly high rate of packed red blood cell (pRBC) transfusions [1]. Up to 90 % of neonates less than 1 kg at birth require at least one packed red blood cell (pRBC) transfusion prior to discharge [1,2].
While erythropoietic stimulating agents [3], the practice of delayed cord clamping or umbilical cord milking [4], and restrictive transfusions guidelines [5,6] have led to a decrease in pRBC transfusion rates, the frequency and total number of transfusions remain high.
Though still controversial a Cochrane review [7] of early erythropoietin reported increased stage >3 retinopathy of prematurity and on this basis did not recommend early erythropoietin administration.
Strategies to prevent transfusion within the first week of life may provide immediate benefit and further allow time for late erythropoietin to become effective.
pRBC transfusions are associated with adverse neonatal outcomes
Complications of pRBC transfusions have been widely described in adults. Recently there have been increased reports of complications of pRBC transfusion administered to premature infants.
Recent evidence indicates that pRBC transfusions are associated with necrotizing enterocolitis (NEC) and intraventricular hemorrhage (IVH). A number of observational studies have demonstrated an association between pRBC transfusion and NEC [8], a condition that typically occurs at several weeks of life.
It has been hypothesized that NEC following pRBC transfusion follows a distinct pathological pathway. This is supported by the observation that a subset of NEC occurs within 72 hours following pRBC transfusion. It is plausible that heavy blood loss in the first days of life may contribute to this pathway.
The observation of NEC within 72 hours of pRBC transfusion has not been studied in prospective randomized controlled trials and therefore remains controversial. IVH is a condition encountered in critically ill and premature infants. IVH typically occurs within the first week of life, a time in which an infant experiences significant blood loss from phlebotomy.
Avoiding transfusion during the first week of life may be meritorious. Recent reports associate transfusions with IVH. One study [9] by Christensen et al. demonstrated a more than 50 % decrease in severe IVH from 17 % to 8 % following institution of restrictive transfusion guidelines.
Moreover, the rate of IVH was 27 % among infants receiving a transfusion in the first week of life compared to 2 % among those not receiving a transfusion. Due to the observational nature of this study, causation cannot be concluded. However, this finding compels one to consider IVH rates and the effect that lower phlebotomy loss in the first days of life could produce.
Use of cord blood for admission laboratory testing is one method to dramatically decrease phlebotomy loss during this critical period.
Use of cord blood at delivery for initial admission laboratory testing
Admission laboratory testing in premature and other critically ill infants commonly includes a complete blood count (CBC) with manual white cell differential, blood culture, blood type and screen, and mandated newborn metabolic screen.
Some institutions may obtain additional laboratory studies routinely. Chromosomes or other genetic testing is also occasionally necessary. Even without genetic testing, routine tests typically require 3 or more mL of blood.
This blood volume represents a significant percent of a critically ill, premature neonate’s total circulating blood volume (Table I). Because these laboratory tests are done immediately after birth, they can be successfully obtained from cord blood in over 90 % of neonates, including neonates receiving delayed cord clamping or cord milking at birth [10].
| Test volume (mL)* | Percent of circulating blood volume | |||||
| Infant birth weight | ||||||
| 500 g | 750 g | 1000 g | 1500 g | 2500 g | ||
| Blood culture | 1 | 2,5% | 1,7% | 1,3% | 0,8% | 0,5% |
| CBC/diff | 0,3 | 0,8% | 0,5% | 0,4% | 0,3% | 0,2% |
| Type and screen | 1 | 2,5% | 1,7% | 1,3% | 0,8% | 0,5% |
| Newborn metabolic screen | 0,6 | 1,5% | 1,0% | 0,8% | 0,5% | 0,3% |
| Chromosomes/microarray | 3 | 7,5% | 5,0% | 3,8% | 2,5% | 1,5% |
| TOTAL | 5,9 | 14,8% | 9,8% | 7,4% | 4,9% | 3,0% |
TABLE I: Percent of circulating blood volume required for laboratory testing in neonates of various birth weight
1. Complete blood count
Obtaining a CBC with manual white cell differential is important in critically ill infants to assess risk of anemia, thrombocytopenia, and infection. Obtaining cord blood can provide these results without necessitating additional phlebotomy from the infant.
To date there are two studies comparing CBC results from paired samples of cord blood and blood from the neonate. Hansen et al. examined the correlation of I/T ratio between cord blood and neonatal blood in term infants.
They demonstrated the correlation in paired samples of white blood cell (WBC) count, hematocrit, and platelets (r=0.70, 0.54, 0.65, respectively) [11]. More recently a study of premature infants compared paired samples in a preterm population [12]. This study demonstrated a high level of agreement between cord blood samples compared to those obtained directly from the infant (Table II).
However, when the cord blood platelet count was less than 100,000/µL, there was a poor correlation with the neonatal platelet count, suggesting that clotting of the cord blood may occur before sampling in some cases, and that low platelet counts from cord blood should be repeated after birth.
This study also reported that the mean WBC count, though not paired samples, from different neonatal sources varied significantly (i.e., capillary, radial arterial, umbilical venous, umbilical arterial), leading one to question whether currently accepted sources for the admission CBC should be interpreted with the same reference values or have their own source-specific reference values.
| Fetal cord blood | Neonatal admission blood | Difference (95 % CI)a |
|
| White blood cell count (× 1,000 cells/µL) |
|||
| Capillary (n=18) | 9.5 | 14.1 | 4.6 (3.1, 6.0) |
| Radial artery (n=25) | 8.6 | 11.4 | 2.8 (1.5, 4.0) |
| Umbilical venous catheter (n=58) |
8.3 | 9.5 | 1.2 (0.5, 1.9) |
| Umbilical arterial catheter (n=38) |
7.4 | 10.3 | 2.9 (1.7, 4.1) |
| All sources (n=139) | 8.3 | 10.7 | 2.4 (1.9, 2.9) |
| Hemoglobin (g/dL) | |||
| Arterial and venous specimen (n=146) |
14.5 | 15.1 | 0.6 (0.3, 0.9) |
| All sources (n=174) | 14.5 | 15.5 | 1.0 (0.7, 1.2) |
| Platelet count (× 1,000 cells/µL) | |||
| All sources (n=141) | 224 | 230 | 6 (-4, 15) |
TABLE II: Paired comparison of white blood cell, hemoglobin, hematocrit, and platelet count in fetal cord blood and neonatal admission blood.
2. Blood culture
The majority of neonates admitted to the NICU require a blood culture on admission as they often have respiratory distress, sometimes caused by infection. Blood cultures require more blood volume than any other laboratory test commonly done on admission, with genetic testing as a rare exception.
It is common for neonatologists to decrease the blood culture volume to 0.5 mL in the smallest neonates to minimize phlebotomy loss. This strategy increases the risk of false negative results [13]. In contrast, utilizing cord blood immediately after delivery offers clinicians the opportunity to increase the volume of the blood culture, thereby decreasing the chance for a false negative result.
Herson et al. [14] demonstrated a five-fold increase in true positives when the blood culture volume was increased to 5 mL and obtained from the cord blood among high-risk premature infants. Other studies, too, have found an increase, though less dramatic, in the frequency of true positive blood cultures from cord blood (Table III).
Greater sensitivity due to higher volume of blood cultures from cord blood may prevent unnecessary prolongation of antibiotic treatment course. Of great concern to clinicians is a contaminated blood culture from any source. In this regard, it is important to remember that:
- The umbilical cord and fetal side of the placenta is bathed in the same amniotic fluid as the fetus.
- The placenta is delivered in the same manner as the fetus, whether through the vaginal canal or abdominal incision. This exposes the umbilical cord and placenta to the same pathogens as the fetus at the time of delivery.
- Cord blood remains isolated from maternal blood by the trophoblastic membrane.
- Betadine or chlorhexidine must dry to be effective. This is true whether used on the neonate or placenta/umbilical cord.
Remembering these principles will guide those obtaining adequate cord blood cultures.
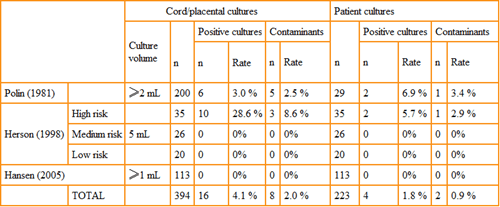
TABLE III: Comparison of blood cultures obtained from the umbilical cord/placenta and postnatal neonatal sampling.
Reprinted from Carroll PD, Widness JA. Nonpharmacological, blood conservation techniques for preventing neonatal anemia – effective and promising strategies for reducing transfusion. Semin Perinatol 2012; 36,4: 232-43, with permission from Elsevier [18].
3. Blood type and screen
The American Association of Blood Banks [15] states, “Initial [neonatal] testing must include ABO and D typing of their red cells and a screen for unexpected red cell antibodies, using either plasma or serum from the infant or mother.” Among tests done on the neonate following delivery, clinicians have the greatest experience with utilizing cord blood for a type and antibody testing.
Blood type and antibody testing can be performed using cord blood. Extreme caution must be taken in obtaining this sample. If the needle is near placental tissue, user error may result in improperly obtaining maternal blood from the intervillous space rather than fetal blood from fetal vessels on the placenta. Discussions with local blood banks can help guide the clinician on when cord blood may be appropriate for type and screen in anticipation of transfusion.
4. Newborn metabolic screen
Each state has established its own requirements for newborn metabolic screening. This testing aims to identify metabolic or genetic conditions that, when identified before symptomatic, can be treated, thereby preventing or delaying complications, including mental retardation and death. Variability exists in the number, frequency, and timing of testing.
This variability increases the challenge with regard to optimal timing of screening. Current guidelines published by the Clinical and Laboratory Standards Institute recommend [16] for premature, low-birth-weight, or sick infants “serial dried blood spot screening with the first screen obtained on admission.”
Obtaining the first newborn metabolic screen from cord blood is accurate for hemoglobinopathies, biotinidase deficiency, galactosemia, and fatty acid oxidation disorders. Other conditions may yield false positive or negative results, whether the sample immediately after birth is from cord blood or blood directly from the neonate.
This is particularly true for cystic fibrosis (false positive) and hypothyroidism (false negative). Repeat testing at a later date is required to more adequately screen for these disorders.
5. Cord pH and blood gas
Obtaining a cord blood gas including pH, oxygen and carbon dioxide measurements can provide valuable information, particularly when there is concern for fetal distress or perinatal asphyxia [17]. Blood from the umbilical artery is the preferred source to assess neonatal status at delivery.
This is due to the fetal circulation pattern of oxygenated blood entering the fetus via the umbilical vein and leaving the fetus via the umbilical artery following oxygen extraction in fetal tissue. Similarly, the pH of umbilical arterial blood is more reflective of fetal acid-base status than umbilical venous blood.
Although it is possible to obtain neonatal admission laboratory tests from umbilical arterial blood, it has been more often obtained from umbilical venous blood. Therefore, arterial cord gasses are generally obtained separately.
Obtaining neonatal admission laboratory studies from umbilical cord blood does not interfere with the ability to obtain umbilical arterial cord blood gas.
Use of cord blood decreases neonatal transfusions
Two studies report using cord blood to replace routine neonatal phlebotomy at the time of admission to the NICU. In 2011 Christensen et al. [18] reported a feasibility study in which 10 infants had all admission laboratory tests performed on cord blood. These infants were matched by birth weight, gestational age, gender, and race with concurrent controls.
Neonates for whom admission laboratory studies came from cord blood were less likely to receive a pRBC transfusion within the first 72 hours of life (1 vs. 5; p=0.14) and within the first 7 days (4 vs. 16 transfusions; p=0.02). This practice also was associated with a lower incidence of IVH (0 vs. 4 cases; p=0.01).
The mean volume of transfused blood was lower in the study arm (21.5 vs. 5.1 mL; p=0.04). This feasibility study led to a larger multicenter study [10] that demonstrated cord labs could be obtained at a high rate (91 out of 96 eligible) among VLBW infants in three NICUs.
In this study, cases were matched with historical controls. Neonates for whom admission laboratory studies were obtained from cord blood had increased hemoglobin levels in the first 24 hours, lower rate of vasopressor use, and fewer pRBC transfusions (Table IV).
The rate of severe IVH (Grade >3) did not reach the level of statistical significance between the study arms (12 % of cases vs. 21 % of controls; p=0.115). These two studies provide compelling evidence that obtaining admission laboratory studies from cord blood immediately after delivery is not only feasible but beneficial. Randomized controlled trials are needed to better establish this practice.
| Cases with delayed clamping or milking (n=36) | Matched controls (n=36) | P-value | Cases with no delayed clamping or milking (n=60) | Matched controls (n=60) | P-value | |
| One or more erythrocyte transfusion(s) | 25% 9/36 |
64 % 23/36 |
<0.001 | 25 % 15/60 |
42 % 25/60 |
0,05 |
| Number of erythrocyte tranfusions per patient (mean;interquartile range) | 0.6; 0 to 0.5 |
2.2; 0 to 4 |
<0.001 | 0.4; 0 to 0.5 |
1.2; 0 to 2 |
0,001 |
TABLE IV: Frequency and number of transfusions with or without obtaining neontanal admission labs from cord blood.
Technique for drawing cord blood
The process for drawing cord blood for admission laboratory studies is quite simple. It is important to remind the obstetrician to clamp the placental side of the umbilical cord so blood does not drain out prior to collection. Cord blood should be obtained shortly after the placenta is delivered.
Several techniques have been described, including using an umbilical cord segment prepared with alcohol [11], or accessing the vessels directly on the placental surface [14]. We recommend preparing the placental surface and base of the umbilical cord with betadine.
We then recommend insertion of an 18-gauge needle with the bevel down attached to a 10 mL syringe into a pre-identified large vein near the umbilical cord insertion on the placenta (Video Link).
Once the blood is collected, it can be transferred in sterile manner into a blood culture bottle (first), EDTA microsampling tube for CBC and type and screen, sodium-heparin tube for chromosomes, if needed, and directly onto filter paper for newborn metabolic screen according to state regulations.
Conclusion
Significant progress has been made in utilizing placental blood for admission laboratory testing to lessen the need for pRBC transfusions in premature and other critically ill newborns. This practice has shown to be practical, feasible, and anatomically/physiologically sound. Placental labs can be obtained even after delayed cord clamping or cord milking.
The potential of placental labs resulting in decreased IVH and/or NEC is encouraging but unproven. Data showing decreased number and frequency of transfusions in the first 7 days of life following utilization of placental labs is compelling. We therefore recommend that those caring for critically ill and premature newborns strongly consider adopting this practice.
Authors
Patrick D. Carroll, MD *, and John A. Widness, MD†
*Section of Neonatology, Dixie Regional Medical Center, Intermountain Healthcare, St. George, UT USA 84770
†Departments of Pediatrics, Roy J. and Lucille A. Carver College of Medicine, The University of Iowa, Iowa City, IA USA 52242
We appreciate Pamela Kling, MD for review of this manuscript and Mark Hart for valuable editorial and secretarial assistance.
References+ View more
- Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. Journal of Pediatrics 1996; 129,5: 680-87.
- Jansen M, Brand A, von Lindern JS, Scherjon S, Walther FJ. Potential use of autologous umbilical cord blood red blood cells for early transfustion needs of premature infants. Transfusion 2006; 46: 1049-56.
- Aher SM, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2012; 9: CD004868.
- Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 2012; 8: CD003248.
- Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics 2005; 115: 1685-91.
- Kirpalani H, Whyte RK, Andersen C, et al. The premature infants in need of transfusion (PINT) study: A randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. Journal of Pediatrics 2006; 149: 301-07.
- Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2012; 9: CD004863.
- Kirpalani H, Zupancic JAF. Do transfusions cause necrotizing enterocolitis? The complementary role of randomized trials and observational studies. Semin Perinatol 2012; 36: 269-76.
- Christensen RD, Baer VL, Lambert DK, Ilstrup SJ, Eggert LD, Henry E. Association, among very-low-birthweight neonates, between red blood cell transfusions in the week after birth and severe intraventricular hemorrhage. Transfusion 2014; 54,1: 104-08.
- Baer VL, Lambert DK, Carroll PD, Gerday E, Christensen RD. Using umbilical cord blood for the initial blood tests of VLBW neonates results in higher hemoglobin and fewer RBC transfusions. J Perinatol 2013; 33, 5: 363-65.
- Hansen A, Forbes P, Buck R. Potential substitution of cord blood for infant blood in the neonatal sepsis evaluation. Biology of the Neonate 2005; 88: 12-18.
- Carroll PD, Nankervis CA, Iams J, Kelleher K. Umbilical cord blood as a replacement source for admission complete blood count in premature infants. J Perinatol 2011.
- Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of Blood Sbmitted for culture in routine practice in a children's hospital. Pediatrics 2007; 119: 891-96.
- Herson VC, Block C, McLaughlin JC, Tetreault J, Eisenfeld LI, Krause PJ. Placental blood sampling: An aid to the diagnosis of neonatal sepsis. Journal of Perinatology 1998; 18,2: 135-37.
- Carson T. Standards for blood banks and transfusion services. 27th ed. Bethesda, MD: AABB 2011.
- Miller JA, Tuerck J, Adams J. Newborn screening guidelines for premature and/or sick newborns; proposed guidelines. Wayne, PA: Clinical and Laboratory Standards Institute 2008.
- Thorp JA, Rushing RS. Umbilical cord blood gas analysis. Obstet Gynecol Clin North Am 1999; 26,4: 695-709.
- Christensen RD, Lambert DK, Baer VL, et al. Postponing or eliminating red blood cell transfusions of very low birth weight neonates by obtaining all baseline laboratory blood tests from otherwise discarded fetal blood in the placenta. Transfusion 2011; 51,2: 253-58.
- Carroll PD, Widness JA. Nonpharmacological, blood conservation techniques for preventing neonatal anemia – effective and promising strategies for reducing transfusion. Semin Perinatol 2012; 36,4: 232-43.
References
- Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. Journal of Pediatrics 1996; 129,5: 680-87.
- Jansen M, Brand A, von Lindern JS, Scherjon S, Walther FJ. Potential use of autologous umbilical cord blood red blood cells for early transfustion needs of premature infants. Transfusion 2006; 46: 1049-56.
- Aher SM, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2012; 9: CD004868.
- Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 2012; 8: CD003248.
- Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics 2005; 115: 1685-91.
- Kirpalani H, Whyte RK, Andersen C, et al. The premature infants in need of transfusion (PINT) study: A randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. Journal of Pediatrics 2006; 149: 301-07.
- Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2012; 9: CD004863.
- Kirpalani H, Zupancic JAF. Do transfusions cause necrotizing enterocolitis? The complementary role of randomized trials and observational studies. Semin Perinatol 2012; 36: 269-76.
- Christensen RD, Baer VL, Lambert DK, Ilstrup SJ, Eggert LD, Henry E. Association, among very-low-birthweight neonates, between red blood cell transfusions in the week after birth and severe intraventricular hemorrhage. Transfusion 2014; 54,1: 104-08.
- Baer VL, Lambert DK, Carroll PD, Gerday E, Christensen RD. Using umbilical cord blood for the initial blood tests of VLBW neonates results in higher hemoglobin and fewer RBC transfusions. J Perinatol 2013; 33, 5: 363-65.
- Hansen A, Forbes P, Buck R. Potential substitution of cord blood for infant blood in the neonatal sepsis evaluation. Biology of the Neonate 2005; 88: 12-18.
- Carroll PD, Nankervis CA, Iams J, Kelleher K. Umbilical cord blood as a replacement source for admission complete blood count in premature infants. J Perinatol 2011.
- Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of Blood Sbmitted for culture in routine practice in a children's hospital. Pediatrics 2007; 119: 891-96.
- Herson VC, Block C, McLaughlin JC, Tetreault J, Eisenfeld LI, Krause PJ. Placental blood sampling: An aid to the diagnosis of neonatal sepsis. Journal of Perinatology 1998; 18,2: 135-37.
- Carson T. Standards for blood banks and transfusion services. 27th ed. Bethesda, MD: AABB 2011.
- Miller JA, Tuerck J, Adams J. Newborn screening guidelines for premature and/or sick newborns; proposed guidelines. Wayne, PA: Clinical and Laboratory Standards Institute 2008.
- Thorp JA, Rushing RS. Umbilical cord blood gas analysis. Obstet Gynecol Clin North Am 1999; 26,4: 695-709.
- Christensen RD, Lambert DK, Baer VL, et al. Postponing or eliminating red blood cell transfusions of very low birth weight neonates by obtaining all baseline laboratory blood tests from otherwise discarded fetal blood in the placenta. Transfusion 2011; 51,2: 253-58.
- Carroll PD, Widness JA. Nonpharmacological, blood conservation techniques for preventing neonatal anemia – effective and promising strategies for reducing transfusion. Semin Perinatol 2012; 36,4: 232-43.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Cord blood gas analysis in obstetrical practice
Webinar presented by Jan Stener Jørgensen, MD PhD, Head of Obstetrics and Professor of Clinical Obstetrics, University of Southern Denmark Watch the webinarRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar











