Printed from acutecaretesting.org
July 2015
POCT: Taking control in uncontrolled premises
Point-of-care testing (POCT), or near-patient testing (NPT), is a term used to describe laboratory testing performed usually by non-laboratory staff – mainly medical and nursing staff outside the main laboratory.
Its complexity ranges from simple dipstick test to sophisticated analyzer test. Advances in technology have led to the development of analyzers and kits designed for use in this role and which are able to provide an increasing repertoire of tests.
Analytical tests are now available for use in operating theaters, hospital wards, or outpatient departments in the acute sector, in general practice surgeries and in the homes of patients in primary care.
International standard ISO 22870, Point-of-care testing (POCT) – Requirements for quality and competence, defines POCT as: “testing that is performed near or at the site of a patient with the result leading to possible change in the care of the patient” [1].
POCT already covers a broad range of pathology and non-pathology testing. The volume of POCT testing is rapidly increasing with an annual growth rate of 12 % to 15 %.
The point-of-care testing (POCT) has evolved as an important part of laboratory medicine by virtue of its compactness, portability, and the feasibility of operation by non-laboratory personnel, where fast and accurate testing methods are a primary concern and, as a result, improving the patient care [2].
The reasons for performing tests in this setting include convenience to the clinicians, a faster turnaround time (TAT), and advantage to the hospital administration in terms of cost savings.
Concerns that have arisen with the POCT include problems with ensuring quality, potential conflicts of interest, and an uncertainty of the responsibility [2]. In larger networks, testing may be performed at locations ranging from the emergency room (ER), the operating room (OR), or intensive care units (ICUs) in the hospital to satellite outpatient clinics.
Most of these areas are outside the control of central laboratory or any proper governing body. The large majority of clinical staff members involved in POCT are focused primarily on clinical care and are much more variable in their familiarity with the testing process and quality control requirements.
Training and ongoing competency maintenance of the staff performing POCT can be overwhelming to manage. Getting control of this uncontrolled unsupervised area is difficult.
Considering the growing field of POCT it is now time for all the stakeholders to manage all aspects of POCT in a systematic and professional manner and to take control over this area. We have tried an approach with a systemic management of POCT facility and focus on
- Planning
- Building a structured POCT unit
- Implementation
- Proposal to Introduce New POCT Device/Process
- Quality
- Accreditations
PLANNING PROCESS FOR POCT STAKEHOLDERS
The following things should be carefully evaluated when planning a POCT facility:
- Needs of the users
- Cost-benefit analysis
- Literature including evaluations of suitable analyzers
- Availability of internal quality control (IQC) and external quality assessment (EQA)
- Technical support and training from analyzer suppliers
- Life-time of consumables
- Storage facilities for consumables; such as space and temperature
- Competency of staff
Prior to purchase of POCT equipment, it is recommended that all those involved in POCT – and POCT stakeholders – are part of the planning process. This group (in a hospital or clinic setting) might include representatives from the following groups:
- End Users (to state their needs and wants)
- Biomedical Engineering (information on analyzer design issues)
- Information Technology staff (advice on software interfaces and functionality)
- Organizational Quality staff (registration, certification requirements)
- Purchasing/Contracts officer (optional, depending on local practice)
- Representative from local Pathology Laboratory
BUILDING A STRUCTURED POCT UNIT
The first step in taking control over a POC facility involves building an interdisciplinary POC management team, including the laboratory, physicians, and nurses. The interdisciplinary concept occurs in three areas of the hospital: the clinical user unit, the laboratory and the information technology department (ITD)
A typical design of POCT network can be arranged as shown in Fig. 1
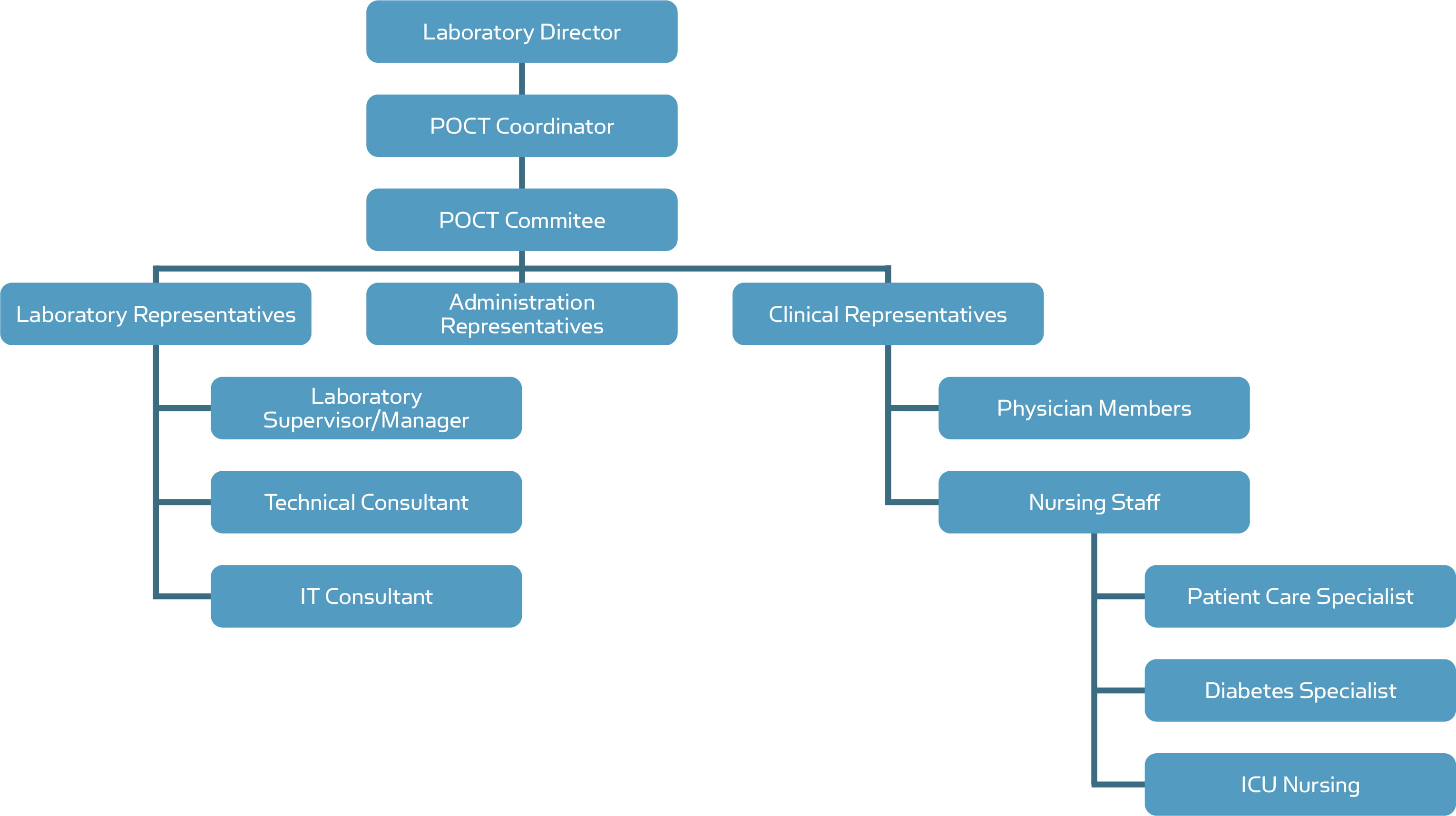
Fig. 1. POCT network
LABORATORY DIRECTOR
All POCT is typically under the direction, authority, and jurisdiction of the Chief of Pathology and Laboratory Medicine.
Responsibilities of the Director of Clinical Laboratory:
- Responsible for all POCT activities
- Compliance with all applicable regulations, rules and standards
- Expert advice and information to the POCT Committee, including identifying alternatives to various POCT methods and devices, determining criteria for medical necessity, and identifying procedures for adopting and implementing the tests
THE ROLE OF DIRECTOR OF THE CLINICAL LABORATORY OR AUTHORIZED REPRESENTATIVE: [4]
- The duty of director includes screening, recommending and approving all instruments, devices, procedures, reagents, materials and kits used in POCT, including new lots of previously approved reagents, and supplies, and new versions of any established POCT
- Appropriate guidelines for quality assessment processes to be established and reviewed regularly
- Interdepartmental collaboration with Medical Staff, Nursing Services, and the Department of Education and Training, in the training of individuals designated as trainers and supervisors of the personnel selected to perform POCT
- Conducts periodic reviews of POCT performance by monitoring for compliance with established guidelines and providing, as required, proficiency test specimens to each site authorized by the Clinical Laboratory to perform POCT
- To oversee periodic inspection of the POCT sites for compliance with regulatory mandates
- Monitors use of all POCT, communicates with each POCT site, and recommends methods to improve efficiency to the unit performing the tests and to the POCT Committee
- Maintains a current master list of all POCT sites and the types of tests performed at each site
ROLE OF THE LOCAL HOSPITAL PATHOLOGY LABORATORY
The local hospital pathology laboratory should play a key role in the development and management of a POCT service. This is particularly true for secondary care and may also be useful for some primary care services.
The pathology laboratory should provide advisory services on multiple issues including the purchase of devices, training, interpretation of results, troubleshooting, quality control, quality assessment and health and safety.
There should therefore be communication between users and the local hospital pathology laboratory on all issues relating to POCT.
Wherever possible this communication should be formally defined, e.g. by a service level agreement specifying the range of products, services, operational details and the responsibilities of the central laboratory and the POCT user.
FOR ANALYZERS IN POCT
For analyzers used in POCT these important factors should be kept in mind:
1. All POCT devices adopted should: [3]
- Be used in accordance with manufacturer or supplier instructions
- Be subject to regular maintenance as specified by the supplying manufacturer
- Details of maintenance performed, faults and corrective actions taken, will be documented
- Only be used for the purpose it has been evaluated and procured for
- Be approved for use by the appropriate local management who will be accountable for the integration of the POCT service into the established POCT quality assurance and operational infrastructure
3. Electronic rather than paper will be used whenever possible. (It is recommended that where such visual read devices are in use, the results are checked by at least two trained staff members).
4. Only trained, certified and competent staff will use POCT equipment. Staff training and certification will be arranged in conjunction with the laboratory POCT team.
5. All reagent/cartridge/consumable lot numbers will be recorded to facilitate patient tracking in the event of product recall. A direct link must be maintained between patient demographics, test results, and reagent identification numbers.
6. All adverse events relating to POCT will be reported back to the Trust Risk Management Team and the POCT Committee by local POCT service users using the Trust Incident Report form. The POCT Committee will have the authority to withdraw or suspend service in the event of: safety-related or performance issues, or lack of clinical or cost effectiveness.
POCT COORDINATOR [3]
POCT should be introduced in a systematic process which is inclusive of all stakeholders. Ad hoc approaches are potentially expensive and dangerous in terms of patient safety. To avoid this situation, a POCT Coordinator should be employed.
An experienced medical professional should be appointed as POCT coordinator
As many people are involved in the creation, implementation and management of a POCT service, it is vital to appoint a POCT Coordinator at the beginning of development process.
This individual will be responsible for both generating results and the correct use of the POC devices. Managers of POCT should also be aware of their responsibility for clinical governance and of the medico-legal implications of an erroneous result.
Liability under the Consumer Protection Act will remain with the manufacturer or supplier if the user can demonstrate that the equipment has been used in strict accordance with the manufacturer’s instructions.
The role of the POCT Coordinator:
- Identifying suitable POCT equipment for evaluation
- Performing an evaluation
- Installing POCT equipment
- Writing procedures
- Training staff
- Preparing worksheets, log books, etc.
- Maintenance schedules
- QC programs
- Troubleshooting
- Monitoring and reviewing procedures
- Competency reviews
ESTABLISHMENT OF A POCT COMMITTEE [3]
In addition to the appointment of a POCT Coordinator, the establishment of a multidisciplinary POCT Committee to oversee POCT whether in the hospital setting or in some elements of primary care is recommended.Representation from all the stakeholders should be included in POCT Committee, e.g. laboratory staff, clinicians, nursing staff, specialty nurses, pharmacists, IT and finance.
Input from a clinical scientist or a biomedical scientist may also be helpful.
The role of the POCT Committee may include the following:
- Determining if POCT is justified at a particular location. This would include a clear demonstration of increased clinical effectiveness
- Establishing a system for the continuing audit and assessment of POCT
- Ensuring that no POCT device is used unless it has been looked at by the POCT committee
- Setting up a quality hierarchy to ensure that there is a direct link between the person performing the analysis and the POCT Committee
- Establishing the presence of a link nurse or other healthcare professional at the point of service delivery
- Including representatives from primary care and the community where necessary
- Ensuring that users have documented training in the use of POCT devices and that they are fully aware of all contraindications and limitations
- Ensuring that IQC and EQA schemes are applied to POCT in the same way as they would be for the established laboratory service
OTHER STAKEHOLDERS
Clinical Consultant
Physicians and Nurse Practitioners within the office or clinic serve as a liaison between the laboratory and clients in reporting and interpreting results.
Technical Consultant
The hospital laboratory is responsible for technical and scientific oversight of the POCT.
- Notifies the Clinical Laboratory upon the arrival of new lots of specified POCT reagents, devices, kits and supplies (e.g., occult blood cards, glucose strips, urine dipsticks, urine pregnancy test kits, etc.).
- Notifies the Clinical Laboratory upon receiving any shipment of specified POCT materials, supplies or devices.
- Provides periodic information on utilization and consumption to the Director of the Clinical Laboratory or authorized representative.
On-Site Supervisor
Nurse Directors, Nurse Managers, and Administrative Clinical Leaders qualify as supervisors based on their education. They will be responsible for the day-to-day supervision or oversight of personnel performing POCT and reporting test results.
A supervisor must be accessible to testing personnel at all times during testing.
FOR THE POCT USERS IT IS IMPORTANT THAT [4]
- All staff must use the equipment in a safe and responsible manner
- All staff must have a unique operator ID
- Operator IDs must remain confidential
- An accurate and up-to-date maintenance log for the POCT equipment must be maintained, signed and dated as required
- All staff members must satisfy the quality control (QC) requirements pertaining to the specific instrument
- All patient and QC results must be documented. Included with the results should be the operator's initials and the date and time of the test.
- All staff members operating POCT equipment will have up to date competency records
- IDs and passwords must remain confidential
- All printouts and transcript results should be signed and dated
- New staff members should receive the necessary POCT training from the POCT Coordinator or a credited staff member.
References+ View more
- ISO 22870:2006. Point-of-care testing (POCT) – Requirements for quality and competence.
- Handorf CR, ed. Alternate site laboratory testing. In: Clinics in Laboratory Medicine. Philadelphia, USA, WB Saunders Co., 1994; 14: 451-645.
- International Federation of Clinical Chemistry Document. Thinking of Introducing PoCT – Things to Consider. 20 March 2014.
- Richard W.C. Pang Point-of-care testing (POCT): Whose responsibility? JHKMTA 1997/98; 7: 9-13
References
- ISO 22870:2006. Point-of-care testing (POCT) – Requirements for quality and competence.
- Handorf CR, ed. Alternate site laboratory testing. In: Clinics in Laboratory Medicine. Philadelphia, USA, WB Saunders Co., 1994; 14: 451-645.
- International Federation of Clinical Chemistry Document. Thinking of Introducing PoCT – Things to Consider. 20 March 2014.
- Richard W.C. Pang Point-of-care testing (POCT): Whose responsibility? JHKMTA 1997/98; 7: 9-13
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








