Printed from acutecaretesting.org
September 2004
Quality assurance of the preanalytical phase - complying with ISO 15189:2003
Introduction
Laboratory medicine practice includes several measures to assure high-quality laboratory services. Traditionally, clinical laboratories have focused their attention on quality control methods and quality assessment programs dealing with analytical aspects.
However, a growing body of evidence has been accumulated in the last decades to demonstrate that quality in clinical laboratories cannot be assured simply by focusing on purely analytical aspects [1].
In fact, an important framework for patient safety and improved laboratory services is the Total Testing Process (TTP). In this cyclical process a patient or physician initiates testing to answer a clinical or public-health question.
The laboratory test is ordered, the patient identified, and the specimen is collected, transported and prepared for analysis. The specimen is then analyzed and the results are interpreted and reported to the physician or the persons who ordered the tests.
Finally, action is taken based on the interpretation of the test results (Fig. 1). A recent review of errors in laboratory medicine concluded that in delivery of laboratory testing, mistakes occur more frequently before the laboratory does the test (preanalytic) or after the test has been performed [2].
Many of the mistakes in the TTP are referred to as laboratory error, but are actually due to poor communication, actions by others involved in the testing process (physicians, nurses, phlebotomists, etc.) or poorly designed processes outside of the laboratory’s control [3].
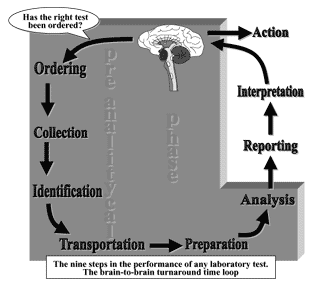
FIG. 1. The Total Testing Process and the preanalytical phase.
The recent availability of an International Standard specifically developed for clinical laboratories, that is the ISO 15189:2003 “Medical laboratories: particular requirements for quality and competence”, allows laboratory professionals to implement a quality system that takes into consideration all steps and processes included in the simplified three-phase (pre-, intra- and postanalytical phase) framework.
According to this systematic approach, both quality and competence issues should be considered in implementing and documenting any process.
The preanalytical phase
According to the ISO 15189:2003 International Standard, pre-examination processes include “steps starting in chronological order from the clinician’s request, including the examination requisition, preparation of the patient, collection of the primary sample, transportation to and within the laboratory and ending when the analytical examination starts”.
From a theoretical viewpoint, the preanalytical phase can be further subdivided into two parts: the first is the so-called “pre-preanalytical phase”, in which the clinician decides which laboratory test should be performed on the basis of his/her knowledge and experience; the second is the “conventional” preanalytical phase that involves a series of related processes starting from patient identification, through the choice of right collection tubes, ending with sample transportation and preparation for analysis [4].
The pre-preanalytical phase includes the formulation of a clinical question and the selection of the appropriate examinations.
The inappropriate utilization of laboratory services is under scrutiny worldwide both for possible effects on total costs and for increased risk of medical errors and injury.
Therefore, the importance of the provision of consultant advice as part of a laboratory service to improve appropriateness is unanimously recognized.
There are large variations in the estimates of inappropriate laboratory use, ranging from 11 to 70 percent for general biochemistry and hematology tests, 5 to 95 percent for urine screens and microbiology, 17.4 to 55 percent for cardiac enzymes and thyroid tests [5].
A large number of studies have been conducted on interventions to reduce the overuse and inappropriate use of laboratory tests.
Combinations of interventions are more effective than single interventions and, in particular, the implementation and diffusion of evidence-based laboratory guidelines should be associated with a continuous monitoring and clinical advice granted by laboratory specialists [6].
The conventional preanalytical phase includes the practical steps of ordering, collection and handling, transportation and reception of samples prior to the examination itself.
A central issue in these practical steps is maintaining the integrity of the relationship between the primary sample(s) and the patient, and between the primary sample(s) and the request documentation, and then finally, in the preparation process, the relationship between the primary sample and secondary preparations from the primary sample.
In forensic medicine these relationships are evocatively, but effectively, referred to as a “chain of custody” [7].
A basic prerequisite, however, is the quality of the primary sample(s) that has to be collected in a standardized way, using appropriate materials, at specific times or after specific preparation of the patient.
Complying with ISO 15189:2003
ISO 15189:2003 clause 5.4 'Pre-examination procedures' includes requirements for a request form, a primary sample collection manual, the traceability of primary samples to an identified individual (the patient), monitoring of samples in transport, recording of receipt of samples, processing of urgent samples and policies for rejection of samples.
According to these requirements the clinical laboratory has to assure “the right test, the right order, to the right patient, for the right question”.
Request form
Request (or requisition) forms (whether hard copy or electronic) from clinicians and the reports issued by the laboratory are the most important means of communication.
In ISO 15189:2003, clause 5.4.1, the 'Pre-examination procedures' section requires that the request form have space for the inclusion of certain items of information.
In addition to a minimum of information (name of the patient, date of birth, identification number and date of collection) that is absolutely needed to allow a complete and unambiguous identification of the patient, physicians should provide an added value to their requests by indicating the clinical question and other information on the patient, thus allowing the laboratory professionals to select the most appropriate tests or test cascade.
Advantages and problems related to electronic request of laboratory tests are well addressed in various papers.
In particular, it should be underlined, the potential role of ward order systems in encouraging clinicians to select the most appropriate tests, in facilitating dissemination of protocols and guidelines and in making possible a real-time consultation by phlebotomists regarding specimen type, sample timings and any other information useful for a state-of-the-art specimen collection [8].
Sample collection manual
The various tests ordered may require one or more different specimen types, e.g. serum, plasma, whole blood and/or urine. The importance of a collection manual is, therefore, evident.
Information in this manual should be checked whenever the phlebotomist is unsure of the proper specimen.
In particular, the manual should contain information regarding the appropriate tube(s), possible anticoagulant of choice, amount of blood to be collected, possible need of immediate refrigeration and any other aspect that can affect the ultimate quality of testing.
Identification and collection
The mechanism by which the specimen is associated with the patient and the request card is of utmost importance.
The introduction of “positive specimens identification” with unique identification labels (barcodes) and reduction of transcription errors has significantly decreased the risk of errors in the preanalytical phase.
In the case of blood gas analysis, for example, arterial blood is needed. Arteries are more difficult to access because they are buried deep in the tissue, and the arterial pressure is greater than the venous pressure.
Damage to the artery is more serious than damage to a vein and could require surgery to repair. For these reasons, arterial punctures require extensive training and practice.
Special syringes are required and an immediate transport, preventing or slowing down the metabolic processes by rapidly cooling the specimens, and fast analysis should be granted.
All this information, including the level of competence required of phlebotomists, should be documented in the collection manual.
Specimen transportation
Problems related to this phase fall into two categories; those associated with the timely and safe delivery of the specimen to the laboratory in a fit condition for examination, and those concerned with the health and safety of all personnel who might come in contact with the specimen, or its container, in transit [9].
Both portering services and pneumatic tube systems present advantages and disadvantages, risks and associated problems that require specific standard operating procedures and staff training.
Specimen reception
ISO 15189:2003 requires that “all primary samples received shall be recorded in an accession book, worksheet, computer or comparable system’ and that ‘the date and time of receipt of samples, as well as the identity of the receiving officer, shall be recorded”.Policy for urgent samples
Specimen preparation includes all those activities that are required to get a sample into a condition suitable for analysis on plasma or serum.
This includes centrifugation, aliquoting, pipetting, dilution and sorting of the specimens into batches for introduction to automated analyzers [9].
The specimen preparation step has attracted considerable attention both for the recognized risk in terms of hazard for laboratory staff, and for the significant contribution to total cost and time of testing (turnaround time)[10] (Fig. 2).
The introduction of automated preanalytical processing units has been found effective in reducing of the labor associated with specimen processing, in decreasing the number of laboratory errors that occur with specimen sorting, labelling and aliquoting.
Furthermore, these instruments improve the integrity of specimen handling throughout the steps of specimen processing and the safety of laboratory staff [11].
Another means to improve turnaround time may be to use point-of-care instruments measuring on whole blood where applicable.
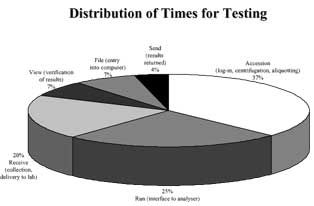
FIG. 2. Distribution of time for testing (from reference 10, modified)
Acceptance/rejection criteria
ISO 15189:2003 requires that “criteria shall be developed and documented for acceptance or rejection of primary samples” and “if compromised primary samples are accepted, the final report shall indicate the nature of the problem and if applicable, that caution is required when interpreting the result”.
Specimens or samples can be compromised by uncertain identity (e.g. a request card received with an inadequately labelled specimen container in the same plastic envelope) or by inadequacy of the specimen (e.g. analysis vitiated by hemolysis).
Mechanisms for categorizing and recording these incidents will enable corrective and/or preventative action be taken.
Discussion
ISO 15189:2003 identifies several requirements for quality and competence in the preanalytical phase of laboratory testing but it does not specify quality indicators and related quality specifications.
While there is a consensus on analytical quality specifications, only recently some indicators and specifications for the preanalytical phase have been proposed. For example, quality indicators have been identified for requests, sampling, transport and receiving samples [12,13].
The related quality specifications, or limits of acceptability, derive from a literature review and reflect the present situation (state-of-the-art), but they have to be verified in the routinary practice.
In particular, it should be demonstrated that monitoring these indicators and related specifications leads to reduced error rates and improved clinical outcomes.
Conclusion
Quality in the preanalytical phase of laboratory activity can be assured by implementing a quality system that complies with the ISO 15189:2003 requirements.
In particular, this International Standard, specifically developed for medical laboratories, takes into account both quality issues and the competence needed to deliver a state-of-the-art laboratory service; however, it lacks to specify specific quality indicators.
References+ View more
- Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997: 43: 1348-51
- Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem 2001; 48: 691-98.
- Plebani M, Bonini P. Interdepartmental cooperation may help avoid errors in medical laboratories. BMJ 2002; 324: 42324.
- Stroobants AK, Goldschmidt HMJ, Plebani M. Error budget calculations in laboratory medicine: linking the concepts of biological variation and allowable medical errors. Clin Chim Acta 2003; 333: 169-76.
- Silverstein MD. An approach to medical errors and patient safety in laboratory services. A white paper. The Quality Institute Meeting, Atlanta, April 2003.
- Solomon DH, Hashimoto H, Daltroy L, Liang MH. Techniques to improve physicians’ use of diagnostic tests: a new conceptual framework. JAMA 1998; 280: 2020-27.
- Burnett D. A practical guide to accreditation in laboratory medicine. London, ACB Venture Publications, 2002.
- Jones R, O’Connor J. Information management and informatics: need for a modern pathology service. Ann Clin Biochem 2004; 41: 183-91.
- Miller JJ. Specimen collection, handling, preparation, and storage. In Clinical Diagnostic Technology: The total testing process (Volume 1: The preanalytical phase). Ward-Cook KM, Lehman CA, Schoeff LE, Williams RH Eds.
- Godolphin W, Bodker K, Uyeno D, Goh LO. Automated blood-sample handling in the clinical laboratory. Clin Chem 1990; 36: 1551-55.
- Holman JW, Mifflin TE, Felder RA, Demers LM. Evaluation of an automated preanalytical robotic workstation at two academic centers. Clin Chem 2002; 48: 540-48.
- Plebani M. Towards quality specifications in extra-analytical phases of laboratory activity. Clin Chem Lab med 2004; 42: 576-77
- Ricos C, Garcia-Victoria M, de la Fuente B. Quality indicators and specifications for the extra-analytical phases in clinical laboratory management. Clin Chem Lab Med 2004; 42: 578-82.
References
- Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997: 43: 1348-51
- Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem 2001; 48: 691-98.
- Plebani M, Bonini P. Interdepartmental cooperation may help avoid errors in medical laboratories. BMJ 2002; 324: 42324.
- Stroobants AK, Goldschmidt HMJ, Plebani M. Error budget calculations in laboratory medicine: linking the concepts of biological variation and allowable medical errors. Clin Chim Acta 2003; 333: 169-76.
- Silverstein MD. An approach to medical errors and patient safety in laboratory services. A white paper. The Quality Institute Meeting, Atlanta, April 2003.
- Solomon DH, Hashimoto H, Daltroy L, Liang MH. Techniques to improve physicians’ use of diagnostic tests: a new conceptual framework. JAMA 1998; 280: 2020-27.
- Burnett D. A practical guide to accreditation in laboratory medicine. London, ACB Venture Publications, 2002.
- Jones R, O’Connor J. Information management and informatics: need for a modern pathology service. Ann Clin Biochem 2004; 41: 183-91.
- Miller JJ. Specimen collection, handling, preparation, and storage. In Clinical Diagnostic Technology: The total testing process (Volume 1: The preanalytical phase). Ward-Cook KM, Lehman CA, Schoeff LE, Williams RH Eds.
- Godolphin W, Bodker K, Uyeno D, Goh LO. Automated blood-sample handling in the clinical laboratory. Clin Chem 1990; 36: 1551-55.
- Holman JW, Mifflin TE, Felder RA, Demers LM. Evaluation of an automated preanalytical robotic workstation at two academic centers. Clin Chem 2002; 48: 540-48.
- Plebani M. Towards quality specifications in extra-analytical phases of laboratory activity. Clin Chem Lab med 2004; 42: 576-77
- Ricos C, Garcia-Victoria M, de la Fuente B. Quality indicators and specifications for the extra-analytical phases in clinical laboratory management. Clin Chem Lab Med 2004; 42: 578-82.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








