Printed from acutecaretesting.org
July 2009
Clinical aspects of the anion gap
CONCEPT OF THE ANION GAP - ITS DEFINITION AND CALCULATION
Blood plasma is an aqueous (water) solution containing a plethora of chemical species including some that have a net electrical charge, the result of dissociation of salts and acids in the aqueous medium. Those that have a net positive charge are called cations and those with a net negative charge are called anions; collectively these electrically charged species are called ions.
The law of electrochemical neutrality demands that, in common with all solutions, blood serum/plasma is electrochemically neutral so that the sum of the concentration of cations always equals the sum of the concentration of anions [1].
This immutable law is reflected in FIGURE 1, a graphic display of the concentration of the major ions normally present in plasma/serum. It is clear from this that quantitatively the most significant cation in plasma is sodium (Na+), and the most significant anions are chloride (Cl-) and bicarbonate HCO3-.
The concentration of these three plasma constituents (sodium, chloride and bicarbonate) along with the cation potassium (K+) are routinely measured in the clinical laboratory as part of the most frequently requested biochemical profile, urea and electrolytes (U&E).
FIGURE 1: Normal ionic anatomy of serum [19]
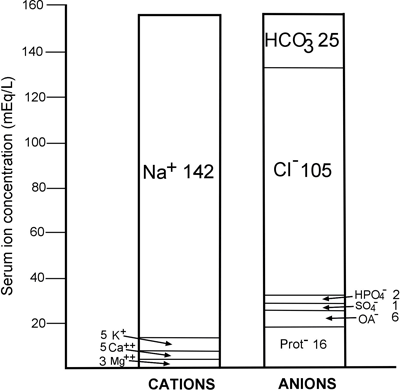
It is clear from FIGURE 1 that the concentration of the main cation sodium (142 mmol/L) exceeds the combined concentrations of the two principal anions, chloride and bicarbonate (130 mmol/L) by 12 mmol/L.
This is the anion gap, which is defined as the difference between plasma/serum sodium concentration and the sum of plasma/serum chloride and bicarbonate concentrations [1]:
| Anion gap (AG) (mmol/L) = [Na+] - ([Cl-] + [HCO3-]) Definition 1 | |
| Where | [Na+] = the plasma/serum sodium concentration (mmol/L) |
| [Cl-] = the plasma/serum chloride concentration (mmol/L) | |
| [HCO3-] = the plasma/serum bicarbonate concentration (mmol/L) | |
An alternative, less frequently used definition of anion gap allows inclusion of the cation potassium (K+):
Anion gap (AG) (mmol/L) = ([Na+] + [K+]) - ([Cl-] + [HCO3-]) Definition 2
Thus for an individual with the following plasma electrolyte values:
| Plasma sodium | 139 mmol/L |
| Plasma potassium | 4.0 mmol/L |
| Plasma chloride | 103 mmol/L |
| Plasma bicarbonate | 26 mmol/L |
| The anion gap is | EITHER 139 - (103 + 26) = 10 mmol/L if definition 1 is used |
| OR (139 + 4.0) - (103+26) =14 mmol/L if definition 2 is used. |
Unfortunately, there remains no consensus about which of the two definitions is adopted, but for accurate interpretation of patient results it is obviously important to know which of the two definitions has been used. For the remainder of this article definition 1 will be used.
The calculated anion gap is an artificial construct that merely reflects the fact that the concentration of the commonly measured cations exceeds the concentration of the commonly measured anions.
The law of electrochemical neutrality demands that overall the anion gap of serum, after taking into account the combined concentration of all unmeasured cations [UC] and all unmeasured anions [UA], must be zero:
Anion gap (AG) = ([Na+] + [UC]) - ([Cl-] + [HCO3-] + [UA]) = 0
It follows from this that the "calculated" anion gap reflects an inequality between the combined concentration of unmeasured anions [UA] and the combined concentration of unmeasured cations [UC], and highlights the important point that calculated anion gap is as much a function of the unmeasured ion concentration as of the concentration of the measured ions (Na, Cl, HCO3) used to make the calculation. From the above equation we can state that:
[Na+] + [UC] = [Cl-] + [HCO3-] + [UA] and by rearrangement
[Na+] - ([Cl-] + [HCO3-]) = [UA] - [UC]
and since Anion gap (AG) = [Na+] - ([Cl- ] + [HCO3-]) then
Anion gap (AG) = [UA] - [UC]
Thus the anion gap may also be defined as the difference between unmeasured cations and unmeasured anions [2,3].
ANION GAP - REFERENCE INTERVAL
There is inconsistency in the literature regarding the anion gap reference interval [3]. Traditionally, the reference interval was widely accepted to be 8-16 mmol/L if potassium is excluded (definition 1) [1-4].
This approximates to 12-20 mmol/L if potassium is included in the AG calculation. The results of several studies [3,5,6] suggest a much lower reference interval of 3-10 mmol/L. This shift in reference interval is generally attributed to change in methodology used to measure sodium and chloride [3]. Flame photometry was once the sole method of measuring sodium, and chloride was measured colorimetrically.
However, since 1990 these techniques have been largely abandoned in favor of measurement by ion-selective electrode. This cannot be the whole explanation because there remains significant difference in calculated anion gap between modern analyzers that all use ion-selective electrodes.
For example, Roberts et al [7] compared anion gap determined using three different analyzers (all utilizing ion-selective electrode) and concluded that the higher traditional reference interval of 8-16 mmol/L was close to being appropriate for two analyzers, but inappropriate for the third.
For this analyzer the lower interval of 3-10 mmol/L was much more appropriate.
Paulson et al [8] also showed that AG differs depending on the measuring system. They computed mean AG of healthy subjects for eight analyzers (all utilizing ion-selective electrode).
The eight means ranged from 5.9 mmol/L to 12 mmol/L. Thus the 8-16 mmol/L reference interval was found to be entirely appropriate for some analyzers, but far too high for others.
It is evident that each laboratory must determine its own reference interval, and physicians cannot assume that the traditional 8-16 mmol/L reference interval, which is still widely quoted in medical texts, is necessarily appropriate for interpretation of patient results in their institution.
DEVIATION IN ANION GAP
Abnormal anion gap is a relatively common occurrence among hospitalized patients, with increased anion gap being far more common than reduced anion gap.
A retrospective study of 6868 sets of serum electrolytes among hospitalized patients [3], for example, revealed incidences of increased and reduced anion gap to be 37.6 % and 2.9 %, respectively.
Since accurate calculation of the anion gap requires accurate measurement of electrolytes, random laboratory errors can give rise to both reduced and increased anion gap. Due consideration should be given to this possibility, if a pathological cause for deviation in anion gap is not evident.
PATHOLOGICAL CAUSES OF REDUCED ANION GAP
It will be evident from the foregoing discussion that reduction of the anion gap will theoretically arise if there is either an increase in unmeasured cations or decrease in unmeasured anions.
At physiological pH the sum of the negative charges on the proteins normally present in serum far exceeds the sum of the positive charges, so that serum proteins are a significant contributor to the totality of unmeasured anions and an insignificant contributor to the totality of unmeasured cations.
In fact, 80 % of the anion gap is due to serum proteins (see FIGURE 1). Since albumin is the most abundant of the predominantly anionic proteins in serum, it is not surprising that reduction of serum albumin (hypoalbuminemia) reduces the anionic gap.
Feldman et al [9] determined that for every 10 g/L reduction in serum albumin, serum anion gap is reduced by 2.3 mmol/L.
Hypoalbuminemia is the most common cause of reduced anion gap [9,10] and some [11,12] have argued that in patient populations such as the critically ill, who frequently suffer hypoalbuminemia, correction of the serum anion gap for serum albumin concentration is necessary for accurate interpretation of serum anion gap.
Abnormal accumulation of an individual serum protein that has a net positive charge (cation) has the potential to cause reduction in anion gap. The immunoglobulin IgG is one such cationic protein.
Patients suffering IgG myeloma produce large quantities of monoclonal IgG (paraprotein) and as a consequence frequently have reduced anion gap. There is a negative correlation between the concentration of monoclonal IgG paraprotein and anion gap [13], and rarely anion gap can actually have a negative value in patients with IgG myeloma [14]. Polyclonal IgG increase, whatever its cause, is likewise associated with reduced anion gap [15].
As with monoclonal IgG paraproteinemia, the higher the IgG the lower is the anion gap. Polyclonal IgG increase is, for example, the cause of the reduced anion gap evident in patients infected with HIV [16].
Theoretically, increase in serum concentration of the cations potassium, calcium or magnesium decreases the anion gap.
The increases seen in clinical practice are rarely sufficient of themselves to reduce anion gap below the limit of the reference interval, so that hyperkalemia, hypercalcemia and hypermagnesemia are not usually considered in the differential diagnosis of reduced anion gap.
However, in patients with hypoalbuminemia, and patients with raised IgG, increase in any of these cations may well contribute to the reduced anion gap.
The cation lithium (Li+), not normally present in serum, is present in the serum of those prescribed lithium carbonate, a drug used to treat bipolar disorder.
Therapeutic dose is associated with a serum lithium concentration of around 1.0 mmol/L, which is not sufficient to materially affect anion gap, but lithium overdose can result in reduced anion gap or even, in cases of severe intoxication, negative anion gap [17]. The principal causes of reduced anion gap are summarized in TABLE 1.
TABLE 1: Causes of low serum anion gap
|
PATHOLOGICAL CAUSES OF RAISED ANION GAP
Theoretically, raised anion gap can result from either a decrease in unmeasured cations or an increase in unmeasured anions. In practice it is almost exclusively the result of increased unmeasured anions derived from metabolic acids. Metabolic acidosis is thus the most common cause of raised anion gap.
The primary abnormality that characterizes metabolic acidosis, whatever its cause, is reduction in serum bicarbonate (HCO3-) concentration. This may be due to increased utilization of bicarbonate in buffering abnormal accumulation of acids, increased loss of bicarbonate from the body, or inadequate regeneration of bicarbonate by the kidneys.
To maintain electrochemical neutrality this reduction in measured anion (bicarbonate) is accompanied by either an increase in serum chloride (Cl-), the only other measured anion, or much more commonly, an increase in unmeasured anions.
In the first case, because the reduction in measured bicarbonate is matched by the increase in measured chloride, the anion gap remains unchanged and normal; this type of metabolic acidosis is called normal anion gap (hyperchloremic) metabolic acidosis. In the second case, because there is an increase in unmeasured anions, the metabolic acidosis is termed increased anion gap metabolic acidosis.
The anion gap has traditionally proved to be of value in the differential diagnosis of metabolic acidosis [18] because, depending on the cause, metabolic acidosis is associated with either raised anion gap or, much less commonly, normal anion gap.
Recent work reviewed by Kraut & Madias [19] has revealed that this utility of the anion gap is not as clear-cut as was once supposed.
The main topic here is the causes of increased anion gap metabolic acidosis, but for completeness the causes of normal anion gap (hyperchloremic) acidosis, all with the exception of diarrhea quite rare, are listed in TABLE 2.
TABLE 2: Causes of normal anion gap (hyperchloremic) metabolic acidosis
|
Abnormal accumulation of any acid that does not contain chloride has the potential to cause increased anion gap metabolic acidosis. As bicarbonate is consumed in buffering the protons (hydrogen cation) of the acid, the anion of the acid accumulates, thereby preserving electrochemical neutrality. Since the accumulating anion is "unmeasured", the anion gap increases.
Accumulation of lactic acid is probably the most common cause of metabolic acidosis. Here reduction in the concentration of bicarbonate resulting from buffering protons of lactic acid is matched by an increase in the concentration of the "unmeasured" anion lactate.
Lactic acidosis occurs when tissues are deprived of oxygen so it can arise in a range of critical illnesses (sepsis, myocardial infarction, etc.), associated with cardiovascular collapse (shock), as well as trauma involving significant blood loss. A number of drugs, most notably the biguanides, metformin and phenformin, as well as alcohol, can cause lactic acidosis.
Increased lactate is such a common cause of raised anion gap, especially among the critically ill, that some have proposed that the readily available anion gap be used as a screening tool for increased lactate in this patient group [20].
Abnormal accumulation of the endogenous keto-acids, β-hydroxybutyric acid and acetoacetic acid, is the cause of the metabolic acidosis (ketoacidosis) that occurs in untreated diabetes and starvation, when in the absence of intracellular glucose fats are mobilized as an alternative energy source.
Here the increased anion gap is due to increased serum concentration of the "unmeasured" anions, β-hydroxybutyrate and acetoacetate.
Decreased excretion of endogenous acids and their anions is the cause of the increased anion gap metabolic acidosis that is frequently a feature of renal failure.
Intoxication with ethylene glycol and methanol causes raised anion gap by virtue of the metabolic acidosis that results from their metabolism to glycolic and formic acid, respectively [21].
Pyroglutamic acidosis is a rare, but probably under-recognized cause of raised anion gap metabolic acidosis [22]. Kortmann et al [23] report three case histories, all occurring in the same institution within a 6-month period.
The ratio of change in anion gap to change in bicarbonate (∆AG: ∆HCO3) can be used to help identify coexisting acid-base disturbances in patients with metabolic acidosis.
This utility of the anion gap is based on the notion that in cases of pure increased anion gap, metabolic acidosis (lactic acidosis, diabetic ketoacidosis, etc.), the increment in anion gap from normal is equal to the decrement in bicarbonate from normal, so that the ratio is 1 [24]. A ∆AG: ∆HCO3 ratio of < 1 (decrease in bicarbonate greater than increase in anion gap) suggests a coexisting normal anion gap (hyperchloremic) acidosis or coexisting compensated respiratory alkalosis.
A ratio of > 1 (increase in AG greater than decrease in bicarbonate) suggests either coexistent metabolic alkalosis or coexistent respiratory acidosis. Kraut & Madias [19] provide a critical review of the validity of this use of the anion gap.
Although metabolic acidosis is almost invariably the cause of marked increase in anion gap (i.e. AG > 25 mmol/L), it is not necessarily the cause in lesser degrees of increase (AG 16-20 mmol/L).
A number of other conditions can give rise to a slight increase in anion gap. Raised serum albumin (hyperalbuminemia) can increase anion gap by the same mechanism that reduced serum albumin (hypoalbuminemia) reduces anion gap [9]. Small increases in anion gap (of the order 4-6 mmol/L) are evident in patients suffering metabolic alkalosis uncomplicated by other acid-base disturbance [25].
This has enabled the use of anion gap in helping to differentiate the raised bicarbonate of primary metabolic alkalosis from the raised bicarbonate that arises during metabolic compensation of respiratory acidosis. In the first case the anion gap is raised and in the second the anion gap is normal.
By a similar mechanism that accounts for the reduced anion gap in patients with IgG myeloma, some patients with IgA myeloma have a raised anion gap [26]. This reflects the observation that some serum IgA paraproteins are anionic, in contrast to serum IgG, which is a cationic protein.
The causes of increased anion gap are listed in TABLE 3.
TABLE 3: Principal causes of raised serum anion gap
|
SUMMARY
The anion gap is an additional piece of clinical information that can be easily derived without cost from the most commonly requested biochemical profile, urea and electrolytes (U&E). It is not possible to calculate the anion gap if serum chloride is not included in the profile.
The principal clinical use of the anion gap is in assessment of acid-base disturbances, particularly metabolic acidosis. Marked increase in anion gap (> 25 mmol/L) is almost invariably incontrovertible evidence of metabolic acidosis.
The distinction between normal and high anion gap metabolic acidosis allows the anion gap to help in determining the primary cause of metabolic acidosis. The anion gap has additional utility in the investigation of patients who present with metabolic acidosis in association with another acid-base disturbance.
Reduced anion gap is by comparison with increased anion gap a rare finding that is not associated with disturbance in acid-base balance but can be indicative of several conditions including hypoalbuminemia, myeloma and lithium intoxication.
Accurate interpretation of anion gap results is potentially hampered by lack of consensus on the method of calculation and significant variability in reference interval.
Laboratory error (either preanalytical or analytical) in measurement of any or all of the three (or four) parameters required to make the calculation can invalidate anion gap results. The anion gap is thus unusually vulnerable to laboratory error.
References+ View more
- Emmett M, Nairns R. Clinical use of the anion gap Medicine 1977; 56: 38-54
- Bartlett D. Understanding the anion and osmolal gaps. Laboratory values: What they are and how to use them. J Emerg Nursing 2005; 31: 109-11
- Lolekha P, Vanavanan S, Lolekha S. Update on value of the anion gap in clinical diagnosis and laboratory evaluation. Clin Chim Acta 2001; 307: 33-36
- Oh M, Carrol H. The anion gap. New Eng J Med 1977; 297: 814-17
- Winter S, Pearson R, Gabow P et al. The fall of the serum anion gap. Arch Int Med 1990; 150: 311-13
- Sadjadi S. A new range for the anion gap. Arch Int Med 1995; 123: 807
- Roberts W, Johnson R. The serum anion gap. Has the reference interval really fallen? Arch Pathol Lab Med 1997; 121: 568-72
- Paulson W, Roberts W, Lurie A et al. Wide variation in serum anion gap measurements by chemistry analyzers. Am J Pathol 1998; 110: 735-42
- Feldman M, Soni N, Dickson B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J Clin Lab Med 2005; 146: 317-20
- Lolekha P, Lolekha S. Value of the anion gap in clinical diagnosis and laboratory evaluation. Clin Chem 1983; 29: 279-83
- Gabow P, Kaehny W et al. Diagnostic importance of an increased serum anion gap. New Eng J Med 1980; 303: 854-88
- Figg J, Jabor A, Kadza A et al. Anion gap and hypoalbuminemia. Crit Care Med 1998; 26: 1807-10
- Mansoor S, Siddiqui I, Adil S et al. Anion gap among patients of multiple myeloma and normal individuals. Clin Biochem 2007; 40: 226-29
- Gumprecht T, O’Connor D, Reardon A. Negative anion gap in a young adult with multiple myeloma. Clin Chem 1976; 22: 1920-21
- Qujeq D, Mohiti J. Decreased anion gap in polyclonal hypergammaglobulinemia. Clin Biochem 2002; 35: 73-75
- Al-Aly Z, Valdez H, Moiz A et al. Evaluation of serum anion gap in patients with HIV. J Nephrology 2007; 20: 727-30
- Sood M, Richardson R. Negative anion gap and elevated osmolar gap due to lithium overdose. CMAJ 2007; 176: 921-23
- Reilly R, Anderson R. Interpreting the anion gap. Crit Care Med 1998; 26: 1771
- Kraut J, Madias N. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2: 162-74
- Berkman M, Ufberg J et al. Anion gap as a screening tool for elevated lactate in patients with an increased risk of developing sepsis in the emergency department. J Emerg Med 2007; 36: 391-94
- Mycyk M, Aks S. A visual schematic for clarifying the temporal relationship between the anion and osmol gaps in toxic alcohol poisoning. Am J Emerg Med 2003; 21: 333-35
- Moe O, Fuster D. Clinical acid-base pathophysiology: disorders of plasma anion gap. Best Prac & Res Clin Endo & Metab 2003: 17: 559-74
- Kortman W, vanAgtmael J et al. 5-Oxoproline as a cause of high anion gap metabolic acidosis: an uncommon cause with common risk factors. Neth J Med. 2008: 66(8): 354-7
- DiNubile M.. The increment in the anion gap: overextension of a concept? Lancet 1988; 2: 951-53
- Madias N, Ayus J et al. Increased anion gap in metabolic alkalosis: role of plasma protein equivalency. New Eng J Med 1979; 300: 1421
- Paladinin G, Sala P. Anion gap in multiple myeloma. Acta Haematol 1979; 62: 148-52
References
- Emmett M, Nairns R. Clinical use of the anion gap Medicine 1977; 56: 38-54
- Bartlett D. Understanding the anion and osmolal gaps. Laboratory values: What they are and how to use them. J Emerg Nursing 2005; 31: 109-11
- Lolekha P, Vanavanan S, Lolekha S. Update on value of the anion gap in clinical diagnosis and laboratory evaluation. Clin Chim Acta 2001; 307: 33-36
- Oh M, Carrol H. The anion gap. New Eng J Med 1977; 297: 814-17
- Winter S, Pearson R, Gabow P et al. The fall of the serum anion gap. Arch Int Med 1990; 150: 311-13
- Sadjadi S. A new range for the anion gap. Arch Int Med 1995; 123: 807
- Roberts W, Johnson R. The serum anion gap. Has the reference interval really fallen? Arch Pathol Lab Med 1997; 121: 568-72
- Paulson W, Roberts W, Lurie A et al. Wide variation in serum anion gap measurements by chemistry analyzers. Am J Pathol 1998; 110: 735-42
- Feldman M, Soni N, Dickson B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J Clin Lab Med 2005; 146: 317-20
- Lolekha P, Lolekha S. Value of the anion gap in clinical diagnosis and laboratory evaluation. Clin Chem 1983; 29: 279-83
- Gabow P, Kaehny W et al. Diagnostic importance of an increased serum anion gap. New Eng J Med 1980; 303: 854-88
- Figg J, Jabor A, Kadza A et al. Anion gap and hypoalbuminemia. Crit Care Med 1998; 26: 1807-10
- Mansoor S, Siddiqui I, Adil S et al. Anion gap among patients of multiple myeloma and normal individuals. Clin Biochem 2007; 40: 226-29
- Gumprecht T, O’Connor D, Reardon A. Negative anion gap in a young adult with multiple myeloma. Clin Chem 1976; 22: 1920-21
- Qujeq D, Mohiti J. Decreased anion gap in polyclonal hypergammaglobulinemia. Clin Biochem 2002; 35: 73-75
- Al-Aly Z, Valdez H, Moiz A et al. Evaluation of serum anion gap in patients with HIV. J Nephrology 2007; 20: 727-30
- Sood M, Richardson R. Negative anion gap and elevated osmolar gap due to lithium overdose. CMAJ 2007; 176: 921-23
- Reilly R, Anderson R. Interpreting the anion gap. Crit Care Med 1998; 26: 1771
- Kraut J, Madias N. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2: 162-74
- Berkman M, Ufberg J et al. Anion gap as a screening tool for elevated lactate in patients with an increased risk of developing sepsis in the emergency department. J Emerg Med 2007; 36: 391-94
- Mycyk M, Aks S. A visual schematic for clarifying the temporal relationship between the anion and osmol gaps in toxic alcohol poisoning. Am J Emerg Med 2003; 21: 333-35
- Moe O, Fuster D. Clinical acid-base pathophysiology: disorders of plasma anion gap. Best Prac & Res Clin Endo & Metab 2003: 17: 559-74
- Kortman W, vanAgtmael J et al. 5-Oxoproline as a cause of high anion gap metabolic acidosis: an uncommon cause with common risk factors. Neth J Med. 2008: 66(8): 354-7
- DiNubile M.. The increment in the anion gap: overextension of a concept? Lancet 1988; 2: 951-53
- Madias N, Ayus J et al. Increased anion gap in metabolic alkalosis: role of plasma protein equivalency. New Eng J Med 1979; 300: 1421
- Paladinin G, Sala P. Anion gap in multiple myeloma. Acta Haematol 1979; 62: 148-52
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









