Printed from acutecaretesting.org
July 2006
Intrapartum asphyxia
Thus, EFM has been used for nearly half a century. EFM was originally expected to prevent intrapartum asphyxia, which hopefully would minimize the incidence of cerebral palsy (CP) in fetuses born at term. However, the incidence of CP has remained nearly unchanged, whereas intervention rates, mostly in terms of cesarian section (CS), have risen dramatically ever since [4].
This is partly due to the fact that EFM alone has a poor specificity, leading to a high number of unnecessary interventions (CS). Therefore, research and development in intrapartum surveillance has focused on finding tools to increase the specificity of EFM.
FBS has proven to increase the specificity and further to prevent the consequences of intrapartum asphyxia [5-6].
In Part I of this paper the history of fetal monitoring is briefly presented. Different origins of intrapartum asphyxia and their possible treatment are described, and the different stages of asphyxia in terms of acid-base changes are presented.
Fetal asphyxia
Presence of oxygen is a precondition of aerobic metabolism (FIGURE 1) and supply of enough energy (ATP) to maintain a sound and healthy state of the fetus – even during labor. During episodes with lack of oxygen supply, metabolism turns anaerobic (FIGURE 2), yielding very little energy from the stores, which are depleted rapidly. Hence, the risk of developing asphyxia and finally fetal brain damage or death is imminent.
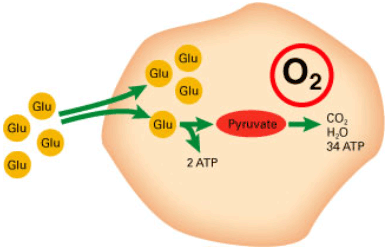
FIGURE 1: Aerobic metabolism of glucose
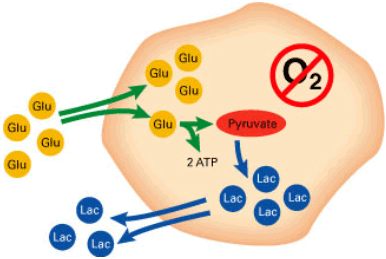
FIGURE 2: Anaerobic metabolism of glucose
The term asphyxia is not defined precisely in the literature, although the condition is well known by obstetricians and neonatologists. To most obstetricians the condition means a state of the fetus with depressed vital functions, usually defined by a fetal acidosis combined with hypoxia/anoxia:
Low pH and low standard base excess: umbilical cord artery (UCA) pH < 7.0 / SBE < -10 mmol/L.
Below these levels there is an increased risk of brain damage [7].
Signs of asphyxia during labor are abnormal heart rate, pathological CTG readings, green meconium-stained amniotic fluid, and low scalp-blood pH.
Asphyxia - physiology and possible treatment
During labor the fetus is highly at risk of encountering periods of hypoxia. Here, the function of the fetal-placental unit as part of the axis between the maternal and the fetal circulation is crucial. "High-risk parts" of this axis are maternal oxygenation and blood pressure, placental function during and in between contractions, umbilical-cord blood flow and fetal circulation.
A "lubricated machinery" ensures the transfer of oxygen from the mother to the fetus, and transfer of CO2 and other waste products from the fetus to the maternal circulation. The placenta, so to speak, serves as a lung for the fetus in utero.
Possible causes of fetal hypoxia/asphyxia during labor
Mechanisms that can alter the function of this axis are: physiological changes during labor like fear, pain and stress, regional anesthesia with concomitant maternal blood pressure changes, too frequent contractions of the uterus – either spontaneous or due to incorrect use of oxytocin to augment the contractions (hyperstimulation) when labor fails to progress (dystocia), placental insufficiency or abruption, and finally different aspects of pregnancy and birth that may alter or compress the blood flow through the umbilical cord. See TABLE 1 for an overview.
| Cause | Effect |
| Maternal hypotension supine position, anesthesia, vasodilation (epidural) |
Utero-placental flow ↓ Fetal pO2/sO2 ↓ |
| Maternal hypoventilation apnea/eclampsia |
Maternal pO2/sO2 ↓ |
| Maternal hyperventilation fear, pain, stress |
Maternal pO2/sO2 ↓ (dead space ventilation) |
| Maternal cathecolamines ↑ (adrenalin ↑) fear, pain, stress |
Utero-placental flow ↓ (from animal experiments) Fetal pO2/sO2 ↓ |
| Uterine hypertonia Hyperstimulation overefficient uterine activity |
Utero-placental flow ↓ Fetal pO2/sO2 ↓ |
| Cord compression oligohydramnios, (maternal) position, breech, cord entanglement, prolapse |
Feto-placental flow ↓ decreased/blocked O2/CO2 exchange |
| Placental abruption/insufficiency | Feto-placental flow ↓ decreased/blocked O2/CO2 exchange |
TABLE 1: Possible causes and effects of fetal hypoxia/asphyxia during labor
Asphyxia - possible treatment
Possible treatment of these high-risk situations for the fetus to avoid severe asphyxia includes: respiratory support and oxygen to the mother, volume substitution to improve maternal blood pressure, positional changes of the mother, anesthesia, cessation or elimination of oxytocin, acute tocolysis (medical inhibition of uterine contractions), amnioinfusion (installation of saline water in the amniotic cavity to relieve pressure on the umbilical cord), and ultimately immediate delivery either by CS or instrumental vaginal delivery. See TABLE 2 for an overview.
| Cause | Treatment |
| Maternal hypotension position, anesthesia, epidural |
Change position, volume substitution vasoconstrictors (ephedrine) |
| Maternal hypoventilation apnea/eclampsia |
Free airways, O2 suppl. (100 %) respiratory instruction/support |
| Maternal hyperventilation (dead space ventilation) |
Eliminate cause (e.g. pain) |
| Maternal cathecolamines ↑ fear, pain, stress |
Analgesia, anesthesia, psychological support, obstetric care |
| Uterine hypertonia/activity Oxytocin hyperstimulation overefficient uterine activity |
Eliminate cause (oxytocin ↓) Change position/tocolysis left lateral position |
| Cord compression oligohydramnios, (maternal) position, breech, cord entanglement, nuchal cord/prolapse |
Change position – amnioninfusion, tocolysis ”lift fetal head” (fill urinary bladder) |
| Placental abruption | Immediate delivery (CAVE tocolysis!!) |
| Placental insufficiency | Cesarian section (preferably after tocolysis) |
TABLE 2: Asphyxia – possible treatment
Normal pH values: During labor
Scalp pH slowly decreases during normal labor, with values between 7.45 and 7.25. No upper limits of normal scalp pH have been described. Values above 7.50 are seldom seen and are probably due to artifacts.
Scalp pH decreases during normal labor [8]:
- First stage: 0.016 pH unit per hour
- Second stage: 0.11 pH unit per hour
– that is, pH drops nearly tenfold faster during the second stage of normal labor with active pushing and bearing-down efforts. This is primarily due to the higher and longer lasting uterine pressure during the second-stage contractions, with concomitant blocked placental perfusion – and due to maternal breath holding. The result is accumulation of CO2 and a rapid fall in pH.
By anoxia (no oxygen supply at all) – e.g. total umbilical-cord compression – pH drops by 0.04 pH unit per minute [9] – e.g. from 7.20 → 6.80 in 10 minutes.
Most European labor wards have a 15-minute limit to the time spent from diagnosing severe asphyxia until delivery of the baby.
It is of utmost importance to realize that these figures count for woman and fetuses in normal labor at term – with normal resources to withstand the physiological stress and burden of labor. In preterm deliveries, or in growth-retarded or overborn fetuses; in clinical situations like pre-eclampsia, infection (e.g. chorioamnionitis), or with placental insufficiency or abruption, the fetus has much fewer reserves, and the risk of fast development of irreversible damage due to severe asphyxia is much higher.
Fetal physiology during labor
When a fetus meets the physiological challenge of labor it has different defense mechanisms to resist the risk of stress and possible development of hypoxia and asphyxia [10].
When a normal delivery turns pathological – e.g. when uterine contractions have been augmented by oxytocin due to failure to progress, and are too frequent (> 5/10 minutes), there are only short intervals in between contractions to restore normal fetal-placental gas exchange: If there is also oligohydramnios and developing cord compression, the fetus uses different mechanisms to withstand. One could schematically divide the phases in the sequence into three:
| I. | The preacidotic period, |
| II. | the period of “stress” with respiratory acidosis and |
| III. | the period of “distress” with metabolic acidosis. |
I. Preacidotic period
- Increasing oxygen utilization (Bohr effect)
- Decreasing activity
- Decreasing growth
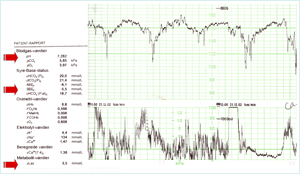
FIGURE 3: CTG and typical scalp-blood gas values in phase I click for enlargement
FIGURE 3 demonstrates the CTG and typical scalp-blood gas values in a situation as described above in I. the preacidotic period, where the fetus meets the challenge of prolonged contractions – and probable cord compression. The CTG can be described as non-reassuring (baseline tachycardia and late decelerations). An FBS has been performed, pH 7.28, pCO2 5.8 kPa, SBE –5.5 mmol/L (lactate 3.5 mmol/L) – and as this is regarded normal, labor continues.
II. Period with “stress” and respiratory acidosis
- Release of stress hormones
- Redistribution of fetal flow
- Anaerobic metabolism in the peripheral fetal tissue
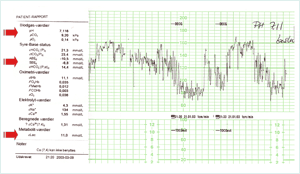
FIGURE 4: CTG and typical scalp-blood gas values in phase II click for enlargement
FIGURE 4 demonstrates the CTG and typical scalp-blood gas values in a situation of fetal stress and respiratory acidotic period. The fetus performs an autoregulation, redistributing the fetal circulation (“centralization” or “brain-sparing”) ensuring flow of oxygen-rich blood to the vital organs by “shutting down” flow to the peripheral non-vital organs.
The CTG can be described as pathological (baseline FHR has dropped and prolonged complicated decelerations), no registration of contractions, but probably they would have been very frequent and long-lasting, leaving very short time in between to ensure oxygen uptake and resulting in CO2 accumulation.
An FBS has been performed: pH 7.11, pCO2 9.2 kPa, SBE –8.8 mmol/L (lactate 11 mmol/L – actually this presents a mixed respiratory-metabolic acidosis) – and is regarded pathological. Acute tocolysis has been performed, and the parturient is taken into the operating theater for an emergency CS.
III. Period with “distress” and metabolic acidosis. Severe asphyxia.
- Anaerobic metabolism in vital organs
- Heart and brain failure
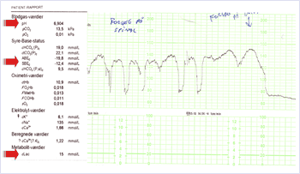
FIGURE 5: CTG and typical scalp-blood gas values in phase III click for enlargement
FIGURE 5 demonstrates the CTG in the operating theater (no registration of contractions) with developing pathology and the period of distress and metabolic acidosis. Initially a slight improvement due to acute tocolysis, left lateral and tilt position, volume substitution and 100 % oxygen to the mother.
The mother had a BMI of 44, and therefore one attempt to perform regional (spinal) anesthesia was carried out – but with no success. CTG shows absent variability, complicated decelerations and finally preterminal bradycardia.
General anesthesia was initiated, and intubation failed for more than 4 minutes. Finally after successful intubation, CS was started, and the baby was delivered less than 60 seconds later. The baby was initially depressed with low Apgar scores and UCA pH 6.98, pCO2 13.5 kPa, SBE – 12.4 mmol/L and lactate of 15 mmol/L (note: pO2 of 0.1 kPa and sO2 of 1.8 %!!).
The baby recovered quickly and was sent home with the mother in good health after 5 days.
Asphyxia during labor, pO2, pCO2, pH, SBE and lactate
To describe, in sequences, the development of severe fetal asphyxia during labor, FIGURE 6 shows the courses of pO2, pCO2, pH, SBE mmol/L and lactate in the three periods:
I. (normal/preacidotic): During normal labor, fetal pO2 values normally lie between 2 and 3 kPa (fetal sO2 30-65 %), pCO2 between 4 and 6.5 kPa, pH: 7.25-7.45. SBE and lactate approx. 0 mmol/L.
II. (stress/respiratory acidosis): When the fetus is stressed due to e.g. prolonged cord compression or too short intervals between contractions, pO2 drops, CO2 accumulates and pH drops rapidly. Fetal autoregulation with centralization of the blood flow to the vital organs results in anaerobic metabolism and accumulation of lactate in the peripheral tissues. There is still enough oxygen left to maintain aerobic metabolism in the vital organs.
Typical values: pO2: 1-1.5 kPa, pCO2: 7-9 kPa, pH: 7.05-7.15 and SBE: –5 to –9 mmol/L, corresponding to lactate values of 5-9 mmol/L.
III. (distress/metabolic acidosis): The oxygen supply in the vital organs of the fetus is now insufficient to maintain aerobic metabolism, lactate accumulates and pH drops rapidly further down below 7.00. Time is short before brain and heart damage occur.
Typical values: pO2: 0.5-1.0 kPa, pCO2: 10-15 kPa, pH: 6.85-6.99 and SBE: –10 to –15 mmol/L, corresponding to lactate values of 10-15 mmol/L.
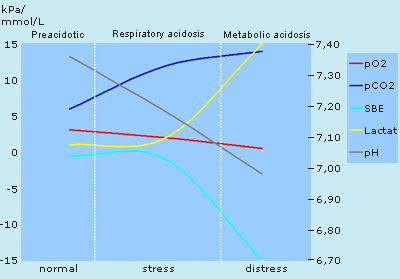
FIGURE 6: Courses of pO2, pCO2, pH, SBE and lactate in the three periods
Summary
To summarize, severe fetal asphyxia during normal labor in healthy term fetuses is rare but have many causes. Different methods to prevent or treat the condition have been described in this paper.
In many cases, threatening intrapartum asphyxia can be recognized in due time and prevented or treated. In part II of the paper, the prognostic consequences of intrapartum asphyxia are discussed, different monitoring possibilities are summarized, and finally an update on current practice in the use of FBS is presented.
References+ View more
- JA Lejemeau de Kergaradec. Quoted by Claude Sureau in "The history of fetal surveillance". In: van Geijn, Copray F (eds). A critical appraisal of fetal surveillance. Amsterdam: Excerpta Medica, 1994: 3-10.
- Hon EH. The electronic evaluation of the fetal heart. Am J Obstet Gynecol 1958; 75: 1215-30.
- Saling E. Neue Untersuchungsmöglichkeiten des Kindes under der Geburt (Einführung und Grundlagen). Geburtshilfe Frauenheilkd 1961; 21: 905-14.
- Neilson JP, Grant AM. The randomised trials of intrapartum electronic fetal heart rate monitoring. In: Spencer JAD, Ward RHT (eds) Intrapartum fetal surveillance RCOG press London 1993: 77-93.
- Jørgensen JS, Wilken-Jensen C, Nickelsen C, Weber T. Fetal scalp blood sampling – a new technique. Acta Obstet Gynecol Scand 1996; 75/Suppl.162: 90.
- Thacker SB, Stroup DF. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev 2000; (Issue no.3).
- Low JA. Intrapartum fetal asphyxia: definition, diagnosis, and classification [see comments]. Am J Obstet Gynecol 1997; 176(5): 957-59.
- Weber T. pH monitoring during labor with special reference to continuous fetal scalp tissue pH. Thesis. Lægeforeningens forlag (eds). Copenhagen 1983.
- Myers RE. Fetal asphyxia due to umbilical cord compression. Metabolic and brain pathologic consequences. Biol Neonate 1975; 26: 21-43.
- Dawes GS. Foetal and neonatal physiology. Chicago: Year Book Medical Publishers, 1968.
References
- JA Lejemeau de Kergaradec. Quoted by Claude Sureau in "The history of fetal surveillance". In: van Geijn, Copray F (eds). A critical appraisal of fetal surveillance. Amsterdam: Excerpta Medica, 1994: 3-10.
- Hon EH. The electronic evaluation of the fetal heart. Am J Obstet Gynecol 1958; 75: 1215-30.
- Saling E. Neue Untersuchungsmöglichkeiten des Kindes under der Geburt (Einführung und Grundlagen). Geburtshilfe Frauenheilkd 1961; 21: 905-14.
- Neilson JP, Grant AM. The randomised trials of intrapartum electronic fetal heart rate monitoring. In: Spencer JAD, Ward RHT (eds) Intrapartum fetal surveillance RCOG press London 1993: 77-93.
- Jørgensen JS, Wilken-Jensen C, Nickelsen C, Weber T. Fetal scalp blood sampling – a new technique. Acta Obstet Gynecol Scand 1996; 75/Suppl.162: 90.
- Thacker SB, Stroup DF. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev 2000; (Issue no.3).
- Low JA. Intrapartum fetal asphyxia: definition, diagnosis, and classification [see comments]. Am J Obstet Gynecol 1997; 176(5): 957-59.
- Weber T. pH monitoring during labor with special reference to continuous fetal scalp tissue pH. Thesis. Lægeforeningens forlag (eds). Copenhagen 1983.
- Myers RE. Fetal asphyxia due to umbilical cord compression. Metabolic and brain pathologic consequences. Biol Neonate 1975; 26: 21-43.
- Dawes GS. Foetal and neonatal physiology. Chicago: Year Book Medical Publishers, 1968.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









