Printed from acutecaretesting.org
April 2006
Reporting calculated GFR from serum creatinine
The National Kidney Disease Education Program (NKDEP) is an initiative of the National Institute of Diabetes and Digestive and Kidney Diseases of the USA National Institutes of Health.
The program promotes public awareness for early detection and treatment of kidney disease to prevent or slow disease progression. The program was initiated because kidney failure is an important public health problem and the incidence of kidney failure and chronic kidney disease (CKD) has risen dramatically in the last 20 years [1].
Glomerular filtration rate (GFR) is considered the best overall indicator of kidney function.
The USA National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) established a classification of stages of CKD severity based on GFR, and defined CKD as either kidney damage or a GFR <60 mL/min/1.73 m2 for 3 months or more, irrespective of cause [2].
The threshold level of GFR <60 mL/min/1.73 m2 was selected as the definition of CKD because at this level about half of an adult’s normal kidney function is lost, resulting in a number of possible complications.
At this threshold GFR, serum creatinine is in the range of 88-140 µmol/L (1.0-1.6 mg/dL) for different demographic groups.
GFR is difficult to measure directly and various procedures to estimate GFR from other laboratory tests have been used. The K/DOQI identified the equation developed from the Modification of Diet in Renal Disease (MDRD) study to give the best agreement with a measured GFR [2].
The MDRD equation was more accurate than a creatinine clearance measurement or the Cockcroft-Gault equation. The MDRD equation requires the serum creatinine, age, gender and race of the individual to estimate the GFR.
Creatinine is a commonly measured laboratory test and the other demographic parameters are readily available. The MDRD equation is suitable for adult individuals 18 years or older, and has been validated in populations of white and African American individuals with GFR less than 90 mL/min/1.73 m2 [FIGURE 1].

FIGURE 1: MDRD equation, IDMS traceable
The MDRD equation has shown good performance for patients with diabetic nephropathy [3] and less satisfactory performance for sick inpatients [4] and for people with near-normal renal function [3]. Validation studies are in progress to include additional ethnic groups and to evaluate alternate estimating equations.
The NKDEP has identified serum creatinine as a primary screening test to identify individuals with CKD. Because the relationship between serum creatinine and GFR is difficult to interpret, particularly at the low creatinine concentrations that correspond to early CKD, the NKDEP recommends that a calculated GFR be reported with all serum creatinine measurements.
This strategy will enable identification of patients early in the course of their kidney disease when therapy can be applied to reduce the progression of CKD and delay or prevent end-stage renal failure.
From its inception, the NKDEP has recognized the global importance of CKD and has included global representation in the program.
A Laboratory Working Group was formed to address the issues of variability in creatinine measurement and to develop a standardization program to reduce the impact of creatinine variability on the utility of the calculated GFR.
The laboratory working group includes global membership and cooperative relationships with professional organizations from other countries.
Recently, the International Federation of Clinical Chemistry and Laboratory Medicine formed a working group for standardization of GFR assessment that works closely with the NKDEP Laboratory Working Group.
PERFORMANCE OF CREATININE MEASUREMENT PROCEDURES
Proficiency testing surveys that included commutable fresh frozen serum samples have reported that most current creatinine methods have a small and variable positive bias when compared to the isotope dilution mass spectrometry (IDMS) reference measurement procedure.
A 2003 survey conducted by the College of American Pathologists (CAP) reported that bias varied from –5.3 to 27 µmol/L (–0.06 to 0.31 mg/dL) among 50 instrument/method peer groups for a sample with a creatinine level of 78 µmol/L (0.90 mg/dL), and that imprecision within a peer group varied from 0.1 to 14.3 % CV [5] [FIGURE 2].
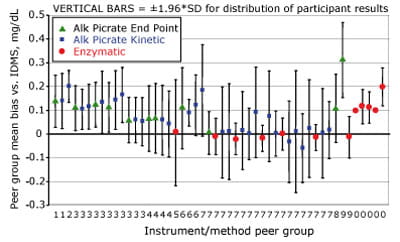
FIGURE 2: Variability in measurement of creatinine @ 0.90
mg/dL (78 µ mol/L)
Similar variability in creatinine measurements was reported in a 2002 International Measurement Evaluation Program (IMEP-17) survey that included many of the same instrument/method groups [6]. In a comparable CAP survey in 1994, routine methods had 9-26 % biases [7].
The impact of creatinine measurement bias and imprecision on the calculation of GFR is more pronounced at low creatinine concentrations corresponding to GFRs near the 60 mL/min/1.73 m2 threshold for identifying CKD, and limits the usefulness of calculated GFR for near-normal kidney function (higher GFRs).
As the GFR decreases, the creatinine concentration increases, and the relative impact of bias and imprecision on the estimated GFR becomes less significant.
The NKDEP recommends not to report a numeric value for calculated GFR above 60 mL/min/1.73 m2 because the bias and imprecision of many routine methods is excessive and the variability in calculated GFR would compromise its clinical utility.
The NKDEP Laboratory Working Group has developed a standardization program for serum creatinine measurements to reduce the bias of creatinine measurements that will, in turn, improve the accuracy and consistency of calculated GFR values.
All methods for measuring serum creatinine should have calibration traceable to an isotope dilution mass spectrometry (IDMS) reference measurement procedure.
The bias needs to be low enough such that, in combination with the imprecision, a routine method’s total error will not cause more than a 10 % influence on the variability of the calculated GFR in the critical creatinine measurement range of 88-140 µmol/L (1.0-1.6 mg/dL).
The report from the Laboratory Working Group includes a figure that represents the necessary combination of bias and imprecision to meet the total error required [8].
Review of the performance of the 50 instrument/method peer groups described previously [5] suggests that 80 % have adequate imprecision to meet the total error goal if the bias could be reduced to low values.
RECOMMENDATIONS OF THE NKDEP LABORATORY WORKING GROUP
The complete Laboratory Working Group recommendations were published in the January issue of Clinical Chemistry [8] and are also available from the NKDEP website (www.nkdep.nih.gov/labprofessionals), which includes the MDRD equations and is updated regularly with the most recent information on recommendations for estimating GFR from serum creatinine and on the creatinine standardization program.
The key recommendations of the NKDEP Laboratory Working Group are summarized here.
-
Laboratories should verify whether their routine creatinine method has been calibrated to IDMS and report calculated GFR with all serum creatinine results, using the appropriate MDRD equation.
The original MDRD equation was developed using creatinine results measured by a routine method that had a small positive bias compared to an IDMS reference measurement procedure. Since many routine methods have a similar bias, this conventional calibration equation is recommended for creatinine results from methods that have not been calibrated to be traceable to IDMS.
A new MDRD equation has been developed for use with creatinine results from methods that have been calibrated to be traceable to IDMS. Both equations are available on the NKDEP website. It is also recommended to use a creatinine value with two decimal places for mg/dL, or to the nearest whole number for µmol/L, units in the MDRD equation to reduce the influence of rounding errors.
- It is recommended to report two values for the estimated GFR, one value if the patient is African American and another value if the patient is not African American. The reason for this recommendation is that race can be difficult to represent reliably in electronic medical records and it is difficult, even if the race is noted, to know if the patient is of a mixed ethnic background.
Consequently, if both values are reported, the physician is able to interpret a value that is appropriate for the patient’s ethnic background.
- If the calculated GFR value is less than or equal to 60, it is recommended to report the value rounded to a whole number (e.g. 53 mL/min/1.73 m2). If the GFR value is greater than 60, it is recommended to report the value as >60 mL/min/1.73 m2.
The reason not to report numeric values greater than 60 is because the impact of variability in the creatinine measurement has a progressively greater impact on the variability of the calculated GFR value as the creatinine value becomes smaller corresponding to more normal renal function, and the accuracy of the estimated GFR is poorer at higher GFRs [3].
It is hoped that the creatinine standardization program will reduce the variability among methods such that it will be possible to reliably report higher values to allow better tracking of patients who may be approaching the threshold of 60 mL/min/1.73 m2.
- Laboratories need to communicate to clinical providers and to pharmacists the clinical issues associated with a creatinine method that is calibrated to be traceable to IDMS.
Because historically all creatinine methods have had a small positive bias, results from an IDMS-traceable method will typically be 10-20 % lower than previously used methods. The critical clinical issues are the change in reference range, and the impact on using creatinine and calculated GFR to adjust dosage of nephrotoxic drugs.
The algorithms used to adjust drug dosages are usually based on the Cockcroft-Gault equation or the absolute creatinine value. Because the product labeling for drugs is based on one of these approaches, pharmacists and providers are obliged to use those algorithms.
Consequently, the laboratory must provide information on the magnitude of difference between an IDMS-traceable creatinine result and a result by the former method used by the laboratory. The pharmacist may use that relationship to “back calculate” a creatinine value that is appropriate for use in a drug-dose algorithm for a nephrotoxic drug.
- In vitro diagnostic (IVD) manufacturers should calibrate creatinine methods to be traceable to an IDMS reference measurement procedure (RMP). There are two principal approaches to establish traceability to a RMP. One approach is to measure native clinical samples, using the routine method and using the RMP.
The product calibrator(s) for the routine method is then value assigned to produce results for the native clinical samples that are equivalent to those from the RMP.
The other
approach to establish the values for the routine method product
calibrator(s) is to use a reference material that is commutable
with the native clinical samples between the RMP and the
routine method, and which has its value assigned by a RMP.
The USA National Institute for Standards and Technology is
collaborating with the NKDEP to produce a new reference
material, NIST SRM 967, that will fill this requirement.
The new SRM is prepared from fresh-frozen off-the-clot serum, has concentrations of approximately 71 and 354 µmol/L (0.8 and 4.0 mg/dL), and is expected to be available in mid-2006. IVD manufacturers may also need to address imprecision and non-specificity of creatinine methods.
- IVD manufacturers should provide information to customers regarding the relationship between results from a creatinine method calibrated to be traceable to IDMS and previous conventionally calibrated methods.
This is essential information that the laboratory needs to make available to clinical providers and to pharmacists.
In addition, IVD manufacturers should provide educational information to laboratories to assist them in reporting calculated GFR and in the transition from conventionally calibrated to IDMS-calibrated routine methods.
- IVD manufacturers should communicate with external quality assurance (proficiency testing) programs to ensure that laboratories participating in those programs are graded appropriately during the transition period from conventionally calibrated to IDMS-calibrated routine methods.
- External quality assurance (proficiency testing) providers should ensure that participants are appropriately graded when there may be a bimodal distribution of results as some laboratories report results using reagent and calibrator inventory that has been conventionally calibrated, while others report results using newer inventory that has IDMS-traceable calibration.
References+ View more
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41: 1-12.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 2002; 39: S1-S246.
- Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 2005; 16: 459–66.
- Poggio ED, Nef PC, Wang X, Greene T et al. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill hospitalized patients. Am J Kidney Dis 2005; 46: 242–52.
- Miller WG, Myers GL, Ashwood ER et al. Creatinine measurement: State of the art in accuracy and inter-laboratory harmonization. Arch Pathol Lab Med 2005; 129: 297-304.
- European Commission - Joint Research Centre, Institute for Reference Materials and Measurements (IRMM). Retieseweg, B-2440. Geel, Belgium: 2003.
- Ross JW, Miller WG, Myers GL, Praestgaard J. The accuracy of laboratory measurements in clinical chemistry. A Study of 11 routine chemistry analytes in the College of American Pathologists Chemistry survey with fresh frozen serum, definitive methods and reference methods. Arch Pathol Lab Med 1998; 122: 587-608.
- Myers GL, Miller WG, Coresh J et al. Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006; 52: 5-18.
References
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41: 1-12.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 2002; 39: S1-S246.
- Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 2005; 16: 459–66.
- Poggio ED, Nef PC, Wang X, Greene T et al. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill hospitalized patients. Am J Kidney Dis 2005; 46: 242–52.
- Miller WG, Myers GL, Ashwood ER et al. Creatinine measurement: State of the art in accuracy and inter-laboratory harmonization. Arch Pathol Lab Med 2005; 129: 297-304.
- European Commission - Joint Research Centre, Institute for Reference Materials and Measurements (IRMM). Retieseweg, B-2440. Geel, Belgium: 2003.
- Ross JW, Miller WG, Myers GL, Praestgaard J. The accuracy of laboratory measurements in clinical chemistry. A Study of 11 routine chemistry analytes in the College of American Pathologists Chemistry survey with fresh frozen serum, definitive methods and reference methods. Arch Pathol Lab Med 1998; 122: 587-608.
- Myers GL, Miller WG, Coresh J et al. Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006; 52: 5-18.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








