Printed from acutecaretesting.org
October 2003
Useful tips to avoid preanalytical errors in blood gas testing: electrolytes
Introduction
For the purpose of this article, a preanalytical error is defined as a deviating result caused by one of the following steps in the preanalytical phase:- Patient preparation
- Blood sampling
- Sample handling
- Sample transport and storage
This article focuses on preanalytical issues concerning the electrolyte parameters available on a modern blood gas analyzer (cNa+, cK+, cCa2+, cCl-) when measured on an arterial whole-blood sample together with the blood gas parameters.
However, the focus of the article is not restricted to this application only, as electrolyte values can also be measured on venous blood, capillary blood, plasma, and serum.
The article does not deal with analytical factors causing a deviation in results or with biological variations.
The article starts by giving a general description of the five main reasons of preanalytical errors when measuring electrolytes and the biochemical background of their influence on measurements.
Next, it provides lists with useful tips on how to avoid preanalytical errors when measuring electrolyte parameters on blood gas analyzers. The lists can be used as checklists when a specific problem is encountered, or as a tool to supplement or expand the knowledge of the staff involved in laboratory medicine, e.g. when updating procedures or when conducting refresher training.
PREANALYTICAL ISSUES - FIVE MAIN REASONS OF PREANALYTICAL ERRORS
The five main reasons of preanalytical errors when measuring electrolytes on a blood gas analyzer are:
- Anti-coagulants
- Sampling from catheters
- Hemolysis
- Storage
- Evaporation
Anti-coagulants
NCCLS [2] recommends the exclusive use of preheparinized sample tubes or syringes for electrolyte measurements on a blood gas analyzer. Other anti-coagulants, e.g. EDTA, can change the pH of the sample slightly as it is a weak acid. Changing pH will also affect the concentration of other parameters in the sample, e.g. cCa2+. In addition, the following issues should be considered:
1.1 Binding effects of heparin
When heparin is added to whole blood it forms a complex with antithrombin III, and the blood's ability to coagulate is inhibited. Heparin also binds all positive ions in blood, especially calcium ions.
To eliminate this effect on calcium, potassium and sodium, some sampling devices come with electrolyte-balanced heparin, which compensates for the binding effect within the normal range of the electrolytes.
An example:
Non-compensated heparin may cause an error on cCa2+ of as much as 6 %. This means that a sample with a true cCa2+ of 1.15 mmol/L will report a value that is 0.07 mmol/L too low - that corresponds to 50 % of the reference range (1.15-1.29 mmol/L) [3].
Samples with a very high or very low cCa2+ may be affected by a small positive or negative bias even with electrolyte-balanced heparin. This effect, however, is only reported to be significant if the syringe is less than 1/3 full [4].
1.2 Dilution with liquid heparin
If heparin is added to the sample in liquid form, the plasma phase of the sample will be diluted, leading to significant deviations of the electrolyte parameters. Therefore dry heparin is recommended [4].
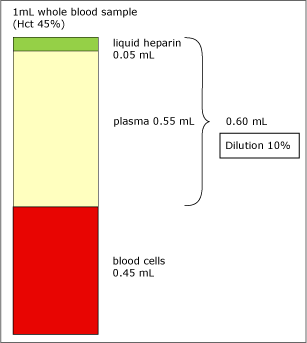
FIG. 1. Dilution of 1 mL whole blood sample (Hct 45 %).
An example:
As Fig. 1 shows, an addition of 0.05 mL liquid heparin to 1 mL whole-blood sample (Hct 45 %) will dilute the plasma phase by 10 %. Since the electrolyte parameters are determined in plasma, the concentrations of these parameters will decrease accordingly.
2. Sampling from catheters
When sampling from catheters, an important aspect to consider is dilution or contamination by flush solution. Flush solutions consist of saline (0.9 % NaCl) with or without heparin added and are present in all catheters to avoid clotting.
When sampling from a catheter, it is important to remove an adequate amount of fluid (also called the discard volume, consisting of flush solution and blood) before sampling to avoid interference and dilution from/by the flush solution on the electrolyte, pH, and blood gas parameters.
The tube volume between the catheter and the sampling port is called the dead space. This volume varies from brand to brand, but the term dead space makes it possible to give a multiple-system guideline.
Arterial catheters
Critically ill patients in an ICU are often equipped with an arterial catheter. Catheters are used for multiple sampling to measure pH, blood gases, and electrolytes. A recent study [5] in this area has shown that to obtain results that are not clinically affected by the flush solution, twice the dead space has to be removed.
The same study showed that to obtain results that are statistically unbiased by flush solution, 5.5 times the dead space has to be removed [5].
2.2 Peripheral venous catheter
Another common way to obtain blood samples from critically ill patients for analysis of the electrolyte status is to use a venous catheter. Venous catheters are used for IV infusions and transfusions.
However, this sampling technique may also cause problems on e.g. potassium measurements, possibly because of hemolysis (caused by shearing forces), hemodilution, or contamination by the intermittent infusions with solutions containing various concentrations of potassium [6].
An example:
A study [6] showed that the difference between cK+ in samples drawn from a peripheral catheter after 3 mL of fluid were discarded and from a venipuncture from the other arm is 0.37 mmol/L. This difference corresponds to approx. 9 % of the reference value for cK+ (3.5-5.0 mmol/L).
3. Hemolysis
Hemolysis causes the release of intracellular components from destroyed erythrocytes (red blood cells) into the extracellular fluid. One of the components is free hemoglobin, which will give the plasma or serum a visible red color when the concentration is above 0.02 g/dL [26].
Some of the components, e.g. potassium (K+), are up to 30 times as concentrated in the intracellular compartment as in the plasma phase. Therefore, the potassium measurement will be highly affected by hemolysis.
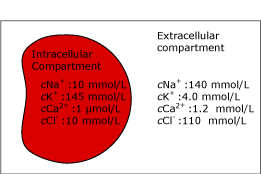
FIG. 2a. The intra- and extracellular compartments. Before hemolysis.
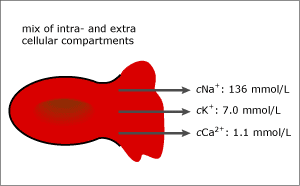
FIG. 2b. 5 % hemolysis (~ 0.8 g/dL free hemoglobin). After hemolysis.
Hemolysis is one of the most frequent preanalytical errors occurring during patient preparation, the sampling process or handling of the sample:
3.1
The erythrocytes will hemolyze if they get in contact with the alcohol used for disinfection of the sampling site [7].
3.2
Intravascular hemolyzation may occur during blood transfusion, depending on the age of the transfused blood [8].
3.3
A very intense aspiration when filling the tube or syringe caused by e.g. obstruction in the needle or too narrow needle size may lead to hemolyzation [9,10].
3.4
Freezing or storage close to the cooling element in the refrigerator may lead to hemolyzation.
3.5
Mixing or shaking the sample too hard may lead to hemolyzation. Older pneumatic tubes may transport the samples too vigorously [8].
The most common causes of hemolysis in capillary sampling of neonatal blood are:
3.6
Squeezing the puncture site during sampling, which may cause hemolysis and/or dilution by tissue fluid [11].
3.7
Mixing the capillary sample too hard with a magnet and a mixing wire/flea may destroy the more fragile red blood cells of a newborn [12,13].
An example:
Hemolysis of as few as 0.5 % of the erythrocytes from a specimen (~0.07 g/dL or 0.05 mmol/L free hemoglobin) can increase the cK+ by 0.5 mmol/L [14]. This corresponds to approx. 12 % of the reference value for cK+ (3.5-5.0 mmol/L). Fig. 3 shows the effect of different degrees of hemolysis on cNa+, cK+ and cCa2+.
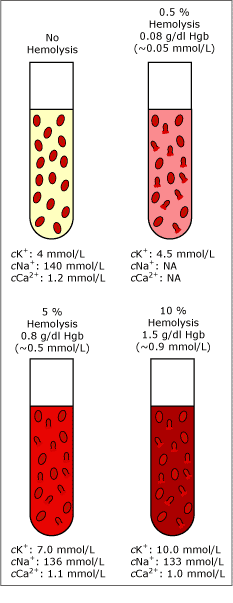
FIG. 3. Degrees of hemolysis. Data combined from [14] and unpublished data by Radiometer Medical A/S.
It should also be mentioned that hemolysis has several lowering effects on plasma cCa2+:
3.8
The calcium ions in plasma are diluted by the intracellular compartment, which has a lower cCa2+.
3.9
Intracellular proteins released into the plasma binds free calcium ions [15].
4. Storage
4.1 Diffusion across the cell membranes
The large difference in intracellular and extracellular cNa+ and cK+ are maintained by the Na+/K+ ATPase pump in the cell membranes. The Na+/K+ ATPase pump is a protein placed in the cell membranes, which actively transports ions across the membrane against the electro-chemical gradient; therefore energy (ATP) is required for the transport.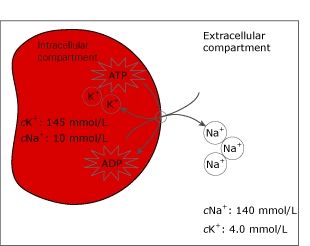
FIG. 4. The Na+/K+-ATPase pump in the cell membrane.
During storage at cool temperatures (0-4 °C) the potassium will leak from the cells, since cool temperatures will inhibit the pump function. This will increase cK+ by 0.1 mmol/L the first hour and by 0.4 mmol/L per hour the following hours [8,16]. Inhibition of the Na+/K+ ATPase pump will also drive sodium into the cells because of the concentration gradient - see Fig. 3 [8].
4.2 Blood-cell metabolism
During storage the blood-cell metabolism continues. The glycolysis, which occurs mainly in the red blood cells [17], is a process where glucose is converted into lactic acid. Consequently, pH will decrease.
The change in pH affects numerous conditions in the sample, among others protein's capacity to bind calcium, since hydrogen ions compete with calcium for binding sites on albumin and other proteins. The rate of glycolysis varies with the hematocrit value of the sample [18].
An example:
A study [19] shows that the rate of calcium increase is about 5.3 % for a pH decrease of 0.1. During a 30-minute storage in gas-tight glass syringes, pH will decrease -0.021 [17], resulting in a minor change of calcium of about 1 %. However, when the sample is stored in plastic syringes and the gases evaporate, pH will decrease even more.
This means that if the general recommendation for storage of samples for blood gas measurement is followed, the diffusion across the cell membrane and cell metabolism can be ignored.
5. Evaporation
Situations in which evaporation may occur:5.1
If the sample is not obtained and stored under anaerobic conditions, evaporation of the water phase, i.e. plasma/serum, will at some point increase the concentration of electrolytes in the sample.
5.2
Evaporation can also occur when a sample is stored opened in a refrigerator. The evaporation increases due to condensation on the cooling element [7].
5.3
An evaporation-like effect can be seen if a sample is centrifuged without a cap. The less dense components of the sample, such as the plasma phase, will be thrown out of the vial. Increased electrolyte values of up to 132 % after centrifugation without a cap has been reported [9].
Conditions such as temperature, airflow, container geometry, and fill volume will influence the effect of evaporation on electrolyte measurements [20].
Useful tips
Below you will find detailed lists of preanalytical issues affecting each of the four electrolyte parameters. The information found in the lists is based on observations reported in various works relating to the preanalytical phase.
The column "due to" refers to the sections in this article. Some of the observations have a larger impact on the measurement result than others. Nevertheless, no observation should be ignored. The lists can be used as checklists for training in the different steps of sampling, or whenever a specific problem is encountered during:
- Patient preparation
- Blood sampling
- Sample handling
- Sample transport and storage
Useful tips when measuring potassium (cK+)
|
Make sure that: |
To avoid: |
Due to: |
|
Patient preparation |
||
|
The patient has not received blood transfusion just prior to the sampling |
cK+ |
Hemolysis |
|
The patient does not clench the fist several times before or during sampling since contracting the forearm cause the release of potassium ions [21] |
cK+ |
|
|
The skin is completely dry if it has been cleaned with alcohol prior to the sampling |
cK+ |
Hemolysis |
|
An appropriate amount of flush solution is discarded prior to sampling from catheters |
cK+ |
Sampling from catheters |
|
Samples are drawn from venous catheters with caution [6] |
cK+ |
Sampling from catheters |
|
The sample is not drawn from an arm where infusion solution is being given or has been given within the last hour [7] |
cK+ |
Sampling from catheters |
|
Blood sampling |
||
|
The needle size is adequate for arterial sampling |
cK+ |
Hemolysis |
|
The catheter or the needle is not partly obstructed by clots during sampling |
cK+ |
Hemolysis |
|
The capillary sample is not obtained by squeezing the puncture site too hard |
cK+ |
Hemolysis |
|
The tourniquet is released after maximum one minute to avoid venous stasis (hemoconcentration), which drives potassium out of the cells [8] |
cK+ |
|
|
Dry electrolyte-balanced heparin is used as anticoagulant |
cK+ |
Anticoagulants |
|
Sample handling |
||
|
The syringe or sampling tube is not mixed too vigorously |
cK+ |
Hemolysis |
|
The mixing of the capillary sample with magnet and mixing wire/flea is not done too vigorously |
cK+ |
Hemolysis |
|
cK+ is measured in plasma, as serum yield results 6.2 % higher due to lysis of the platelets during clotting [7] |
cK+ |
|
|
The sample tube is sealed during centrifugation prior to the measurement |
cK+ |
Evaporation |
|
Sample transport and storage |
||
|
The transportation in the pneumatic tube is not too turbulent |
cK+ |
Hemolysis |
|
The syringe or sample tube is not stored directly on ice |
cK+ |
Hemolysis |
|
The sample is not stored too long at 0-4 °C |
cK+ |
Storage |
|
The sample material is stored in an anaerobic tube or syringe |
cK+ |
Evaporation |
Useful tips when measuring sodium (cNa+)
|
Make sure that: |
To avoid: |
Due to: |
|
Patient preparation |
||
|
An appropriate amount of flush solution is discarded prior to sampling from catheters |
cNa+ |
Sampling from catheters 2.1 |
|
The sample is not drawn from an arm where infusion solution is being given or has been given within the last hour [7] |
cNa+ |
|
|
Blood sampling |
||
|
Dry electrolyte-balanced heparin is used as anticoagulant |
cNa+ |
Anticoagulants |
|
Sample handling |
||
|
The sample is not hemolyzed |
cNa+ |
Hemolysis |
|
The sample tube is sealed if it is centrifuged prior to the measurement [9] |
cNa+ |
Evaporation |
|
Sample transport and storage |
||
|
The sample is not stored too long at 0-4 °C |
cNa+ |
Storage |
|
The sample material is stored in an anaerobic tube or syringe [7] |
cNa+ |
Evaporation |
Useful tips when measuring calcium (cCa2+)
|
Make sure that: |
To avoid: |
Due to: |
|
Patient preparation |
||
|
An appropriate amount of flush solution is discarded prior to sampling from catheters |
cCa2+ |
Sampling from catheters |
|
The sample is not drawn from an arm where infusion solution is being given or has been given within the last hour [7] |
cCa2+ |
|
|
Blood sampling |
||
|
Dry electrolyte-balanced heparin is used as anticoagulant |
cCa2+ |
Anticoagulants |
|
The tourniquet is released after maximum one minute to avoid venous stasis, which can lead to an anaerobic glycolysis with production of lactic acid and decreased pH [8] |
cCa2+ |
Storage |
|
The manufacturer's recommendation on the heparin-to-blood ratio is followed |
cCa2+ |
Anticoagulants |
|
Sample handling |
||
|
The sample is not hemolyzed |
cCa2+ |
Hemolysis |
|
Sample transport and storage |
||
|
The sample is measured within 15 minutes, if stored at room temperature, since glycolysis is increased at room temperature, which can lead to an anaerobic glycolysis with production of lactic acid and decreased pH |
cCa2+ |
Storage |
|
The sample material is stored in an anaerobic tube or syringe |
cCa2+ |
Evaporation |
Useful tips when measuring chloride (cCl-)
|
Make sure that: |
To avoid: |
Due to: |
|
Patient preparation |
||
|
An appropriate amount of flush solution is discarded prior to sampling from catheters |
cCl- |
Sampling from catheters |
|
The sample is not drawn from an arm where infusion solution is being given or has been given within the last hour [7] |
cCl- |
|
|
Blood sampling |
||
|
Dry electrolyte-balanced heparin is used as anticoagulant |
cCl- |
Anticoagulants |
|
Sample handling |
||
|
The sample is not hemolyzed |
cCl- |
Hemolysis |
|
The sample tube is sealed if it is centrifuged prior to the measurement [9] |
cCl- |
Evaporation |
|
Sample transport and storage |
||
|
The sample material is stored in an anaerobic tube or syringe |
cCl- |
Evaporation |
Discussion
Because quality assessment and improvement are focus areas within laboratory medicine and healthcare, many recent articles describe the consequences of medical errors [22,23]. A great part of these errors are related to the preanalytical phase. Some of them go unnoticed, whereas others result in further investigation, repeat sampling, and repeat measurements - all of which add costs to hospitals.To avoid these additional costs and, more importantly, to protect patients, it is necessary to be aware of the consequences of preanalytical errors and to ensure proper procedures and training of staff. With an increasing amount of testing occurring at the point of care, it is even more important to ensure the continuous competence of staff.
In this article the preanalytical errors described are related to the following four steps:
- Patient preparation
- Blood sampling
- Sample handling
- Sample transport and storage
There is no clear limit between deviations arising from physiological and endogenous variables [9,24]. Sometimes there is also doubt whether an error derives from the preanalytical or the analytical phase. Detailed knowledge of different measuring methods affecting the results is therefore also required, e.g. indirect vs. direct measurement of the electrolyte parameters [25].
Conclusion
Determination of the electrolyte parameters is one of the most frequent tests performed in laboratory medicine, both in central laboratories and at the point of care. This article discusses the five main preanalytical errors relating to electrolyte testing and provides useful tips on how to avoid these.
References+ View more
- Bonini P, Plebani M, Ceriotti F et al. Errors in laboratory medicine. Clin Chem 2002; 48,5. 691-98.
- Blonshine S, Alberti R, Olesinski RL. Procedures for the collection of arterial specimens. Approved standard. 3rd ed. NCCLS Publication H11-A3. Wayne, Pa.: NCCLS, 1999; 19, 8.
- Siggaard-Andersen O, Thode J, Wandrup J. The concentration of free calcium ions in the blood plasma "ionized calcium". In: Siggaard-Andersen O, ed. Blood pH, carbon dioxide, oxygen and calcium-ion. 1 ed. Copenhagen: Private Press, 1981: 163-90. Available from Radiometer as AS79, Code No. 918-193.
- Toffaletti J, Ernst P, Hunt P, Abrams B. Dry electrolyte-balanced heparinized syringes evaluated for determining ionized calcium and other electrolytes in whole blood. Clin Chem 1991; 37,10: 1730-33.
- Rickard C, Couchman B, Schmidt S. A discard volume of twice the deadspace ensures clinically accurate arterial blood gases and electrolytes and prevents unnecessary blood loss. Crit Care Med 2003; 31,6: 1654-58.
- Fincher R, Strong J, Jackson J. Accuracy of measurements of hemoglobin and potassium in blood samples from peripheral catheters. Am J Crit Care 1998; 7,6: 439-43.
- Guder WG, Narayanan S, Wisser H et al. Samples: From the patient to the laboratory. 2nd ed. Darmstadt: GIT Verlag GMBH, 2001.
- Narayanan S. The preanalytical phase - an important component of laboratory medicine. Am J Clin Pathol 2000; 113: 429-52.
- Young DS. Effects of preanalytical variables on clinical laboratory tests. 2nd ed. Washington, DC: AACC Press, 1997.
- Thomas L. Haemolysis as influence & interference factor. eJIFCC 2003; 13,4: 1-4.
- Lock, R. The whole-blood sampling handbook. Copenhagen: Radiometer Medical A/S, 2000
- Sommer SR, Warekois RS. Phlebotomy. Philadelphia, Pa: W.B. Saunders Company, 2002.
- Meites S. Pediatric clinical chemistry. Washington DC: AACC Press, 1989.
- Pathology Outreach Client Services Laboratory Handbook. Potassium. http://www.medicine.uiowa.edu/
path_handbook/rhandbook/test1535.html, 2003- Buckley M, Rawson M, Russell J. The effect of haemolysis on ionized calcium measurement. In: Burritt MF, Cormier AD, Maas AHJ, Moran RF, O'Connell KM, eds. Methodology and clinical applications of ion-selective electrodes. Vol. 9. Rochester, MN: Davies Printing Company, 1986: 141-46.
- Christiansen TF. Measurement of potassium in whole blood. Copenhagen: Radiometer Medical A/S, 1983. Available from Radiometer as AS 92, Code no. 918-231.
- Müller-Plathe O. Preanalytical aspects in STAT analysis. Blood Gas News 1998; 7,1: 4-11.
- Ervin K, Azizi K, Chu C, Davis C. The rate of glycolysis in whole blood samples at room temperature (22C). Clin Chem 1995; 41: 201.
- Thode J, Holmegaard SN, Transbøl I, Fogh-Andersen N, Siggaard-Andersen O. Adjusted ionized calcium (at pH 7.4) and actual calcium (at actual pH) in capillary blood compared for clinical evaluation of patients with disorders. Clin Chem 1990; 36,3: 541-44.
- Spandrio L. Pre-analysis variability: the effect of evaporation on the error of measurement, with special reference to sodium and potassium. Quad Sclavo Diagn 1984; 20, 1: 110-21.
- Narayanan S, Guder W. Preanalytical variables and their influence on the quality of laboratory results. eJIFCC 2003; 13,1: 1-5.
- Ehrmeyer SS. The role of training, competency assessment, and continuing education. http://www.acutecaretesting.org/, 2002.
- Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997; 43, 8: 1348-51.
- Young DS. Effects of drugs on clinical laboratory tests. Washington DC: AACC Press, 2000.
- Holbek CC et al. Understanding the different values in electrolyte measurements. http://www.acutecaretesting.org/, 2002.
- Bermes EW, Young DS. General laboratory techniques and procedures. In: Burtis CA, Ashwood ER, 4th ed. Tietz Fundamentals of clinical chemistry. Philadelphia, PA: W.B. Saunders company, 1998: 37-38.
References
- Bonini P, Plebani M, Ceriotti F et al. Errors in laboratory medicine. Clin Chem 2002; 48,5. 691-98.
- Blonshine S, Alberti R, Olesinski RL. Procedures for the collection of arterial specimens. Approved standard. 3rd ed. NCCLS Publication H11-A3. Wayne, Pa.: NCCLS, 1999; 19, 8.
- Siggaard-Andersen O, Thode J, Wandrup J. The concentration of free calcium ions in the blood plasma "ionized calcium". In: Siggaard-Andersen O, ed. Blood pH, carbon dioxide, oxygen and calcium-ion. 1 ed. Copenhagen: Private Press, 1981: 163-90. Available from Radiometer as AS79, Code No. 918-193.
- Toffaletti J, Ernst P, Hunt P, Abrams B. Dry electrolyte-balanced heparinized syringes evaluated for determining ionized calcium and other electrolytes in whole blood. Clin Chem 1991; 37,10: 1730-33.
- Rickard C, Couchman B, Schmidt S. A discard volume of twice the deadspace ensures clinically accurate arterial blood gases and electrolytes and prevents unnecessary blood loss. Crit Care Med 2003; 31,6: 1654-58.
- Fincher R, Strong J, Jackson J. Accuracy of measurements of hemoglobin and potassium in blood samples from peripheral catheters. Am J Crit Care 1998; 7,6: 439-43.
- Guder WG, Narayanan S, Wisser H et al. Samples: From the patient to the laboratory. 2nd ed. Darmstadt: GIT Verlag GMBH, 2001.
- Narayanan S. The preanalytical phase - an important component of laboratory medicine. Am J Clin Pathol 2000; 113: 429-52.
- Young DS. Effects of preanalytical variables on clinical laboratory tests. 2nd ed. Washington, DC: AACC Press, 1997.
- Thomas L. Haemolysis as influence & interference factor. eJIFCC 2003; 13,4: 1-4.
- Lock, R. The whole-blood sampling handbook. Copenhagen: Radiometer Medical A/S, 2000
- Sommer SR, Warekois RS. Phlebotomy. Philadelphia, Pa: W.B. Saunders Company, 2002.
- Meites S. Pediatric clinical chemistry. Washington DC: AACC Press, 1989.
- Pathology Outreach Client Services Laboratory Handbook. Potassium. http://www.medicine.uiowa.edu/
path_handbook/rhandbook/test1535.html, 2003- Buckley M, Rawson M, Russell J. The effect of haemolysis on ionized calcium measurement. In: Burritt MF, Cormier AD, Maas AHJ, Moran RF, O'Connell KM, eds. Methodology and clinical applications of ion-selective electrodes. Vol. 9. Rochester, MN: Davies Printing Company, 1986: 141-46.
- Christiansen TF. Measurement of potassium in whole blood. Copenhagen: Radiometer Medical A/S, 1983. Available from Radiometer as AS 92, Code no. 918-231.
- Müller-Plathe O. Preanalytical aspects in STAT analysis. Blood Gas News 1998; 7,1: 4-11.
- Ervin K, Azizi K, Chu C, Davis C. The rate of glycolysis in whole blood samples at room temperature (22C). Clin Chem 1995; 41: 201.
- Thode J, Holmegaard SN, Transbøl I, Fogh-Andersen N, Siggaard-Andersen O. Adjusted ionized calcium (at pH 7.4) and actual calcium (at actual pH) in capillary blood compared for clinical evaluation of patients with disorders. Clin Chem 1990; 36,3: 541-44.
- Spandrio L. Pre-analysis variability: the effect of evaporation on the error of measurement, with special reference to sodium and potassium. Quad Sclavo Diagn 1984; 20, 1: 110-21.
- Narayanan S, Guder W. Preanalytical variables and their influence on the quality of laboratory results. eJIFCC 2003; 13,1: 1-5.
- Ehrmeyer SS. The role of training, competency assessment, and continuing education. http://www.acutecaretesting.org/, 2002.
- Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997; 43, 8: 1348-51.
- Young DS. Effects of drugs on clinical laboratory tests. Washington DC: AACC Press, 2000.
- Holbek CC et al. Understanding the different values in electrolyte measurements. http://www.acutecaretesting.org/, 2002.
- Bermes EW, Young DS. General laboratory techniques and procedures. In: Burtis CA, Ashwood ER, 4th ed. Tietz Fundamentals of clinical chemistry. Philadelphia, PA: W.B. Saunders company, 1998: 37-38.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Minimizing Pre-analytical errors in blood gas testing
Presented by Ana-Maria Simundic, PhD, Prof. of Medical Biochemistry, Zagreb University, Zagreb, Croatia Watch the webinarRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinarRelated webinar
Evolution of blood gas testing Part 2
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine. Watch the webinarBlood gas Preanalytics app
Get the Blood gas Preanalytics app for your smartphone
This smartphone app focuses on the preanalytical phase of blood gas testing and what operators can do to avoid errors. Download appScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars