Printed from acutecaretesting.org
October 2009
Clinical aspects of pleural fluid pH
PHYSIOLOGY OVERVIEW
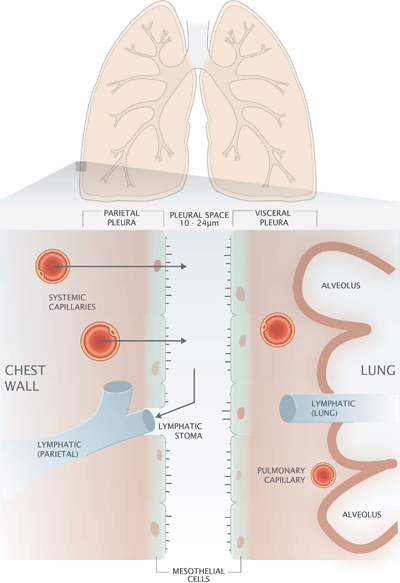
The process of breathing requires mechanical coupling of the thoracic muscles in the chest wall and the lungs. This physiological demand is met by the pleura, a continuous double-layered serous membrane that surrounds the lung and lines the internal surface of the chest wall (FIG. 1).
The inner layer of this membrane system covers the outer surface of the lung and is called the visceral pleura, whilst the parietal pleura is the name given to the outer layer that lines the internal surface of the chest wall.
The potential space between visceral and parietal pleura, called the pleural space, measures just 10-24 µm [1] and is filled with a thin film of liquid called pleural fluid, which is constantly being renewed.
The surface tension created by this thin liquid film is the adhesive force that ensures close apposition and mechanical coupling between lung and chest wall. Pleural fluid also acts as a lubricant preventing friction between pleural surfaces and allowing lung and chest wall to slide with respect to each other during respiratory movements. In health the total volume of pleural fluid around both lungs is estimated to be 0.26 ± 0.1 mL/kg [2].
Thus the volume of pleural fluid surrounding each lung of a healthy adult weighing 75 kg is of the order of 10 mL.
FIGURE 1: Diagrammatic representation of the pleura
In patients with pleural effusion the volume of accumulating pleural fluid and resulting expansion of the pleural space make possible the percutaneous aspiration of pleural fluid by needle inserted between the ribs of the locally anesthetized chest wall, a procedure called thoracentesis [3].
For those with large effusions, thoracentesis can have dramatic therapeutic effect, providing relief from the symptoms of pleural effusion, breathlessness, chest pain and cough.
Since it provides the means for obtaining samples of pleural fluid for laboratory analysis, thoracentesis is also a diagnostic procedure that can be usefully applied to all patients with pleural effusion of unknown cause.
NORMAL PRODUCTION AND COMPOSITION OF PLEURAL FLUID
Histologically, the visceral and parietal pleura are composed of a single layer of microvilli-rich mesothelial cells, sited on a basement membrane and underlying connective tissue that contains a network of blood capillaries and lymphatic vessels (FIG. 1).
These pleural structures are involved in the production and drainage of pleural fluid that ensure a constant volume (approximately 10 mL) of pleural fluid within the pleural space.
Pleural fluid production (approximately 15-20 mL/day) [4] is dependent on the same Starling forces that govern the movement of fluid between vascular and interstitial spaces throughout the body.
The hydrostatic pressure that tends to force fluid out of capillaries is higher in the capillaries of the parietal pleura than in both the pleural space and the capillaries of the visceral pleura. The opposing colloid osmotic (oncotic) pressure, due to protein concentration, is the same in both parietal and visceral pleura capillaries, and much lower in the pleural space.
The net effect of these pressure gradients is continuous movement of an ultrafiltrate of plasma from the capillaries of the parietal pleura to the pleural space [5]. Excess fluid is drained to lymphatics via holes (stomata) in the parietal pleura [5].
Pleural fluid is normally a clear straw-colored fluid rich in hylauronic acid, a lubricating agent elaborated by mesothelial cells. As an ultrafiltrate of plasma, pleural fluid has a low protein concentration (~ 1 g/dL), hence its low oncotic pressure.
Relative to plasma it has a high bicarbonate concentration and high pH (~7.6). Pleural fluid normally contains a small number of red blood cells (~40/µL) and white blood cells (~150/µL), predominantly macrophages (75 %) and lymphocytes (23 %) [2].
CAUSES OF PLEURAL EFFUSION - TRANSUDATE OR EXUDATE?
Pleural effusions occur when more fluid enters the pleural space than is removed [6]; mechanisms of influx and/or efflux may be disturbed. Effusions are classified as either transudates or exudates.
Transudative effusions are the result of a disturbance of the Starling forces (hydrostatic and oncotic pressures) involved in normal pleural fluid production. Increased hydrostatic pressure is the cause of the transudative pleural effusion that occurs in patients with heart failure.
Reduced plasma albumin concentration and consequent reduced plasma oncotic pressure is the cause of the transudative effusion associated with nephrotic syndrome and cirrhosis. In the case of transudative effusions, the pleura is not the source of the problem and remains intact.
By contrast, exudative effusions are usually the result of change (e.g. increased capillary permeability, lymphatic blockage) within the pleura [7]. This loss of pleural integrity and associated exudative effusion can be the result of an infectious, inflammatory or neoplastic disease process, not necessarily (indeed not usually) originating in the pleura.
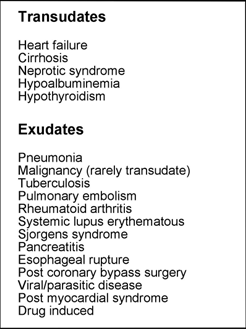
There are many conditions that may be complicated by pleural effusion (TABLE 1) but heart failure, pneumonia, malignant disease and pulmonary embolism are, in order, the four most common causes [7].
TABLE 1: Some causes of pleural effusion
When a patient presents with pleural effusion of unknown cause, the first step is to determine, by laboratory analysis of pleural fluid, whether it is a transudate or exudate.
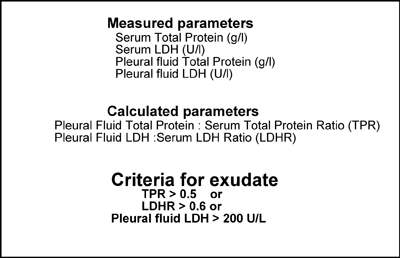
The measurements required to make this distinction are protein concentration and lactate dehydrogenase (LDH) activity of both pleural fluid and blood serum (or plasma). The criteria for an exudate, defined by Light et al [8] are listed in TABLE 2.
If the effusion is found to be transudative, no further laboratory testing of pleural fluid is helpful; the causes are few and would be evident in particular cases from other clinical details. By contrast, exudative effusion has many possible causes and further testing, including measurement of pleural fluid pH, is often useful.
TABLE 2: Lights criteria to distinguish pleural exudate from pleural transudate
PLEURAL FLUID PH - GENERAL CONSIDERATIONS
The only study in which the pH of normal human pleural fluid has been measured returned a value of 7.64 [9]. This is in broad agreement with more recent animal studies [10,11] that suggest that pleural fluid pH normally lies within the range of 7.60-7.66, slightly alkaline compared with blood pH (7.35-7.45). The relatively high bicarbonate concentration of pleural fluid accounts for this difference in pH.
The pH of pleural effusions is almost without exception lower than that of normal pleural fluid and approximates much closer to the pH of blood, with transudative effusions generally having a higher pH (7.45-7.55) than exudative effusions, which with some important exceptions, have a pH in the approximate range of 7.30-7.45 [12].
Highest pleural fluid pH is seen in transudative effusions resulting from heart failure. Light et al [13] measured pleural fluid pH in 178 patients, including 39 with heart failure. In all but four of these 39, pleural fluid pH was >7.4 and ranged from 7.40 to 7.58 (median 7.49).
The four patients with pleural fluid pH <7.4 had an accompanying metabolic or respiratory acidosis, highlighting the fact that acidemia can itself cause a lowering of pleural fluid pH, which may complicate the interpretation of pleural fluid pH results.
All transudative and most exudative effusions have a pH >7.30. Pleural fluid pH <7.30 (termed pleural acidosis) is associated with a limited number of conditions. These are esophageal rupture, tuberculosis, rheumatoid disease, pneumonia and malignant disease.
Of these, esophageal rupture is associated with the lowest pleural fluid pH, usually in the range of 5.0-6.5.
A review of 475 patients with pleural effusion [14] revealed 59 patients with pleural fluid pH <6.0. In all 59 cases the cause of pleural effusion was esophageal rupture. The authors of this review considered a pleural fluid pH of <6.0 virtually diagnostic of esophageal rupture.
So consistent is the finding of pleural acidosis in patients with tuberculosis that some have argued that a pleural fluid pH of >7.4 may be sufficient evidence to exclude a diagnosis of tuberculosis [15].
Although as noted above some patients whose pleural effusion is caused by pneumonia and malignancy have a pleural fluid pH of <7.3, this is by no means always the case; indeed for both of these patient groups it is more common to have a pleural fluid pH of >7.3.
The significance of pleural fluid pH in pneumonia and malignancy warrants more detailed consideration.
PNEUMONIA AND PLEURAL FLUID PH
The second most common cause of pleural effusion after heart failure is pneumonia. Around a third (20-40 %) of patients hospitalized with pneumonia develop an exudative pleural effusion [16]; such effusions are called parapneumonic effusions (PPE).
Three stages in the progression of PPE are recognized [16]. The first is the exudative stage, which begins around 2-5 days after the first symptoms of pneumonia. This is characterized by a rapid outpouring of fluid into the pleural space.
In the absence of effective antibiotic treatment during the subsequent 5-10 days this progresses to the second so-called fibrinopurulent stage in which bacteria gain access and multiply within the pleural space. Large numbers of neutrophils are recruited to the pleural space and fibrin forms due to the entry of intravascular clotting proteins to the pleural space.
Pleural fluid, increasingly rich with bacteria and neutrophils, may become "loculated" within compartments of the pleural space formed by accumulating fibrin strands. Effective treatment at this stage depends on draining the accumulating pleural fluid (therapeutic thoracentesis).
In the absence of such treatment there is progression over the following weeks to the final organizational or empyema stage, which is defined by frank pus within the pleural space and characterized by the growth of a thick fibrous peel over the visceral pleura that ultimately prevents lung expansion. Surgical removal of the peel is necessary for survival.
The metabolic activity of accumulating cells and bacteria within the pleural space causes a progressive fall in pleural fluid pH [17] as PPE progresses from the uncomplicated PPE of the exudative phase through the complicated PPE of the fibrinopurulent stage to empyema.
Thus in the majority of patients with PPE, whose PPE is uncomplicated, pleural fluid pH is >7.30, whereas in patients with empyema pleural fluid pH is always less than 7.3 and usually considerably lower.
In one series of patients with PPE [18], the mean pleural fluid pH of 10 patients with uncomplicated PPE was 7.38 (range 7.33-7.47) compared with a mean of 6.94 (range 6.70-7.21) for patients with complicated ("loculated") PPE. In a separate study, 22 patients with empyema had a mean pleural fluid pH of 6.83 (range 6.29-7.28) [19].
The observation that the lowest pleural fluid pH is associated with the most advanced stage of PPE has led to the widespread use of pleural fluid pH to guide treatment.
All PPE patients require antibiotic therapy, but as the disease progresses to the second fibrinopurulent stage it becomes increasingly necessary to remove (drain) the effusion. Both the American College of Chest Physicians and British Thoracic Society guidelines [20,21] suggest tube drainage in addition to antibiotic therapy for PPE if effusion is culture negative and pH is <7.2.
The corollary of this guideline is that uncomplicated PPE will most likely resolve with antibiotic therapy alone if pleural fluid pH is >7.2. The choice of pH 7.2 as the most appropriate cut-off value is based on a large meta-analysis study of pleural fluid pH in PPE [22]. This study also demonstrated that pleural fluid pH was better able to predict the need for tube drainage than either pleural fluid LDH activity or glucose concentration.
An exception to the general rule that pleural fluids are increasingly acidotic in cases of PPE occurs when the causative bacteria is one of the Proteus species. Only rarely a cause of PPE, these bacteria elaborate the enzyme urease that converts urea to ammonium, rendering pleural fluid markedly alkaline.
In such cases pleural fluid pH is >7.45 and may be as high as 8.0 [23]. In these rare cases pleural fluid pH is diagnostically useful as few, if any, conditions are associated with pH greater than that of normal pleural fluid. However, it would clearly be inappropriate to use the 7.2 pH cut-off to guide the use of drainage therapy in such cases.
Another problem that might confound the interpretation of pleural fluid pH in cases of PPE was highlighted by Maskell et al [24] who demonstrated that the pH of pleural fluid can vary between locules in some patients with complicated "loculated" PPE.
MALIGNANT DISEASE AND PLEURAL FLUID PH
Malignant disease is a common cause of pleural effusion. Although virtually all malignant diseases can be associated with pleural effusion, cancers of the lung, breast and ovary along with lymphoma account for most (75 %) cases of malignant pleural effusion (MPE) [25].
Except in the rare case of mesothelioma (primary tumor of the pleural mesothelium), MPE is a consequence of metastatic spread of tumor cells from a primary site (lungs, breast, etc.) to the pleural space and indicates advanced malignant disease.
Median survival following diagnosis of MPE is just 5 months although there is variation between cancer type; breast cancer, for example, is associated with much longer survival (13 months) than lung cancers (2 months) [26].
Palliative treatment for cancer patients with MPE includes therapeutic thoracentesis, but recurring MPE is treated by pleurodesis, a more invasive procedure in which the pleural space is first drained of all pleural fluid, then obliterated by inducing an inflammatory reaction that fuses parietal and visceral pleura.
Successful pleurodesis prevents the formation of pleural fluid, thereby providing permanent relief from the debilitating breathlessness associated with MPE.
Pleural fluid pH has prognostic significance in cases of MPE that has proved useful in guiding treatment. Good et al [19] determined that the pH of pleural fluid recovered from 44 patients with MPE ranged from 7.04 to 7.55 (median 7.40). A number of studies [26-29] have demonstrated that low pleural fluid pH (<7.3) is associated with reduced survival.
This relative acidosis is assumed to be the result of the metabolic activity of an ever-growing tumor cell mass.
In a retrospective study [26] of 226 patients with MPE, pleural fluid pH ranged from 6.70 to 7.65. For 181 of these patients, whose pleural fluid pH was >7.32, median survival was 6.8 months (range 4.6-9 months), this compared with median survival of just 2.4 months (range 1.1-3.7) for the remaining 45 patients whose pleural fluid pH was <7.32. Sahn et al [27] examined pleural fluid pH in 60 patients with malignant disease.
Median survival of 40 patients with pleural fluid pH >7.3 was 9.8 months compared with just 2.1 months for the remaining 20 patients whose pleural fluid pH was <7.3.The decision to recommend pleurodesis to patients suffering MPE must take account of predicted survival.
The risk and discomfort of this invasive technique, which is by no means always successful, is not justified for patients who have a very short life expectancy; thoracentesis might be the more appropriate treatment.
The observed correlation between pleural fluid pH and survival outlined above has led to the recommendation that pleural fluid pH measurement be used in selecting patients for pleurodesis.
The value of pleural fluid pH in this context is given further credence by studies that have demonstrated that low pleural fluid pH is associated with increased risk of pleurodesis failure [29].
Some authorities [30] have suggested that pleurodesis should not be considered in those with a pleural fluid pH <7.2, but more recent guidelines from the American Thoracic Society/European Respiratory Society [31] suggest that pleural fluid pH should not be the sole criterion in deciding whether pleurodesis is indicated; rather, pleural fluid pH should be considered adjunctive information, one of several factors that need to be considered in selecting patients. This reflects current expert opinion [12].
PLEURAL FLUID PH MEASUREMENT - ANALYTICAL AND PRE-ANALYTICAL CONSIDERATIONS
Despite widespread recommendation that pleural fluid pH should only be determined using a blood gas analyzer [20,21], two other methods, pH meter and pH indicator stick (or litmus paper) are commonly used.
A recent survey conducted in North Carolina [32] revealed that of 11 hospital laboratories measuring pleural fluid pH, just two reported using blood gas analyzers; the rest used either pH indicator stick or more rarely pH meter.
Most (75 %) chest physicians ordering the test in these hospitals wrongly assumed a blood gas analyzer was being used. The results of this survey are broadly consistent with two previous studies [33,34] that suggest that only a third of laboratories use blood gas analyzers.
The evidence that pH meters, pH indicator sticks and litmus paper are insufficiently accurate for measuring pleural fluid pH is contained in a number of studies [33,35,36].
The reluctance of laboratorians to use blood gas analyzers is attributed in part to the fear that pleural fluids may block or damage electrodes because they may contain pus and fibrin clots.
There is no clinical indication for measuring the pH of pleural fluids that contain visible pus because all such effusions require draining, irrespective of the pH. Collecting pleural fluid into heparin-containing syringes prevents clot formation [37].
There is no clearly defined standard method for the collection of pleural fluid for pH measurement. A recent study [37] investigating preanalytical factors that might affect results suggests that pleural fluid should be collected anaerobically into a preheparinized blood gas syringe, ensuring that all air is expelled.
Care should be taken that the sample is not contaminated with even a trace of local anesthetic (lidnocaine) used to prepare the patient for thoracentesis. Analysis should not be delayed beyond an hour after collection.
BULLET POINT SUMMARY
- Pleural effusion, defined as excess fluid in the pleural
space, has many possible causes, the most common are:
- heart failure
- pneumonia
- malignant disease - Pleural effusions are classified as either transudates or exudates
- Normal pleural fluid has a pH of 7.60-7.66
- pH of pleural effusions almost invariably <7.6
- pH of transudates generally higher (7.45-7.55) than exudates (7.30-7.40)
- Pleural acidosis (pleural fluid pH <7.3) occurs when
pleural effusion is the result of:
- esophageal rupture
- tuberculosis
- rheumatoid disease
- malignant disease (in most cases pH >7.3)
- pneumonia (in most cases pH >7.3) - The most important clinical utility of pleural fluid pH measurement is assessment of patients with parapneumonic and malignant pleural effusions
- In patents with parapneumonic effusion, pleural fluid pH <7.2 indicates advanced disease and need for urgent tube drainage in addition to antibiotic therapy
- In patients with parapneumonic effusion, pleural fluid pH >7.2 indicates that antibiotic therapy alone is probably sufficient therapy
- In patients with malignant effusion, pleural fluid pH <7.3 indicates reduced survival and is a contraindication for pleurodesis
- Blood gas analyzers should be used to measure pleural fluid pH - pH indicator sticks and pH meters are not suitable.
- Pleural fluid for pH measurement should be collected anaerobically to a preheparinized syringe and analyzed within 1 hour of collection.
References+ View more
- Paramasivam E, Bodenham A. Pleural fluid collections in critically ill patients. Continuing Education in Anesthesia, Critical Care & Pain 2007; 7: 10-14
- Noppen M, De Waele M, Li R et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med 2000; 162: 1023-26
- Thomsen T, DeLaPena J, Setnick G. Thoracentesis. New Eng J Med 2006; 355 e16
- Light RW. Pleural disease (5th ed). Lippincott Williams & Wilkins: New York 2007
- Zocchi L. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 2002; 20: 1545-58
- Porcel J, Light R. Diagnostic approach to pleural effusions in adults. Am Fam Physician 2006; 73: 1211-20
- Loddenkemper R. Pleural effusion (Chapter 63) In: Clinical Respiratory Medicine 2nd ed. Mosby 2004
- Light R, MacGregor M, Luschinger P, Ball W. Pleural effusion: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507-13
- Yamad S. Uberdieserose flussig keit in der Pleurhohle der gesunden Menschen. Z Ges Exp Med 1933; 90: 409-342-348
- Sahn S, Good T, Willcox M, Potts D. Characteristics of normal rabbit pleural fluid: physiologic and biochemical implications. Lung 1979; 156: 63-69
- Rolf L, Travis D. Pleural fluid-plasma bicarbonate gradients in oxygen toxic and normal rats. Am J Physiology 1973; 224: 857-61
- Sahn S. The value of pleural fluid analysis. Am J Med Sci 2008; 335: 7-15
- Light R, MacGregor M, Ball W, Luschinger P. Diagnostic significance of pleural fluid pH and PCO2. Chest 1973; 64: 591-96
- Dye R, Laforet E. Esophageal rupture: diagnosis by pleural fluid pH Chest 1974; 66: 454-56
- Houston M. Pleural fluid pH: diagnostic, therapeutic and prognostic value. Am J Surg 1987; 154: 333-37
- Light R. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 375-80
- Sahn S, Reller L, Tayle D et al. The contribution of leukocytes and bacteria to the low pH of empyema fluid. Am Rev Respir Dis 1983 128: 811-15
- Potts DE, Levin PE, Sahn S. Pleural fluid pH in parapneumonic effusions. Chest 1976; 70: 326-31
- Good J, Taryle D, Maulitz R et al. The diagnostic value of pleural pH. Chest 1980; 78: 55-59
- Colice G, Curtis A, Deslauriers J et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118: 4-71
- Davies C, Glesson F, Davies R. BTS guidelines for the management of pleural infection. Thorax 2003; 58 (Suppl ii): 18-28
- Heffner J, Brown L, Barbieri C et al. Pleural fluid chemical analysis in parapneumonic effusions: a meta-analysis. Am J Respir Crit Care Med 1995; 151: 1700-08
- Pine J, Holman J. Elevated pleural fluid pH in proteus mirabilus empyema. Chest 1983; 84: 109-11
- Maskell N, Gleeson F, Darby M et al. Diagnostically significant variations in pleural fluid pH in loculated parapneumonic effusions. Chest 2004; 126: 2022-24
- Heffner J, Klein J. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 2008; 83: 235-50
- Bielsa S, Salud A, Martinez M et al. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Int Med 2008; 19: 334-36
- Sahn S, Good J. Pleural fluid pH in malignant effusions: diagnostic prognostic and therapeutic implications. Ann Intern Med 1988; 108: 345-49
- Rodriguez-Pandero F, López Mejias J. Survival time of patients with pleural metastatic carcinoma predicted by glucose and pH studies. Chest 1989; 95: 320-24
- Aelony Y, Yao J, King R. Prognostic value of pleural fluid pH in malignant epithelial mesothelioma after talc poudrage. Respiration 2006; 73: 334-39
- Rodriguez-Pandero F, Antony V. Pleurodesis: state of the art. Eur Respir J 1997; 10: 1648-54
- Antony V, Loddenkemper R, Astoul P et al. Management of malignant pleural effusions. Eur Respir J 2001; 18: 402-19
- Bowling M, Chatterjee A, Conforti J. Perceptions vs. reality: measuring of pleural fluid pH in North Carolina. NC Med J 2009; 70: 9-13
- Chandler T, McCoskey E, Byrd R et al. Comparison of the use and accuracy of methods for determining pleural fluid pH. South Med J 1999; 92: 214-17
- Kohn G, Hardie W. Measuring pleural fluid pH: high correlation of a handheld unit to a traditional tabletop blood gas analyzer. Chest 2000; 118: 1626-29
- ChengDS, Rodriguez R, Rogers J et al. Comparison of pleural fluid pH obtained using blood gas machine, pH meter, and pH indictor strip. Chest 1998; 144: 1368-72
- Lesh E, Roth B. Is pH paper an acceptable, low-cost alternative to the blood gas analyzer for determining pleural fluid pH? Chest 1997; 112: 1291-92
- Rahman N, Mishra E, Davies H et al. Clinically important factors influencing the diagnostic measurement of pleural fluid pH and glucose. Am J Respir Crit Care Med 2008; 178: 483-90
References
- Paramasivam E, Bodenham A. Pleural fluid collections in critically ill patients. Continuing Education in Anesthesia, Critical Care & Pain 2007; 7: 10-14
- Noppen M, De Waele M, Li R et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med 2000; 162: 1023-26
- Thomsen T, DeLaPena J, Setnick G. Thoracentesis. New Eng J Med 2006; 355 e16
- Light RW. Pleural disease (5th ed). Lippincott Williams & Wilkins: New York 2007
- Zocchi L. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 2002; 20: 1545-58
- Porcel J, Light R. Diagnostic approach to pleural effusions in adults. Am Fam Physician 2006; 73: 1211-20
- Loddenkemper R. Pleural effusion (Chapter 63) In: Clinical Respiratory Medicine 2nd ed. Mosby 2004
- Light R, MacGregor M, Luschinger P, Ball W. Pleural effusion: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507-13
- Yamad S. Uberdieserose flussig keit in der Pleurhohle der gesunden Menschen. Z Ges Exp Med 1933; 90: 409-342-348
- Sahn S, Good T, Willcox M, Potts D. Characteristics of normal rabbit pleural fluid: physiologic and biochemical implications. Lung 1979; 156: 63-69
- Rolf L, Travis D. Pleural fluid-plasma bicarbonate gradients in oxygen toxic and normal rats. Am J Physiology 1973; 224: 857-61
- Sahn S. The value of pleural fluid analysis. Am J Med Sci 2008; 335: 7-15
- Light R, MacGregor M, Ball W, Luschinger P. Diagnostic significance of pleural fluid pH and PCO2. Chest 1973; 64: 591-96
- Dye R, Laforet E. Esophageal rupture: diagnosis by pleural fluid pH Chest 1974; 66: 454-56
- Houston M. Pleural fluid pH: diagnostic, therapeutic and prognostic value. Am J Surg 1987; 154: 333-37
- Light R. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 375-80
- Sahn S, Reller L, Tayle D et al. The contribution of leukocytes and bacteria to the low pH of empyema fluid. Am Rev Respir Dis 1983 128: 811-15
- Potts DE, Levin PE, Sahn S. Pleural fluid pH in parapneumonic effusions. Chest 1976; 70: 326-31
- Good J, Taryle D, Maulitz R et al. The diagnostic value of pleural pH. Chest 1980; 78: 55-59
- Colice G, Curtis A, Deslauriers J et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118: 4-71
- Davies C, Glesson F, Davies R. BTS guidelines for the management of pleural infection. Thorax 2003; 58 (Suppl ii): 18-28
- Heffner J, Brown L, Barbieri C et al. Pleural fluid chemical analysis in parapneumonic effusions: a meta-analysis. Am J Respir Crit Care Med 1995; 151: 1700-08
- Pine J, Holman J. Elevated pleural fluid pH in proteus mirabilus empyema. Chest 1983; 84: 109-11
- Maskell N, Gleeson F, Darby M et al. Diagnostically significant variations in pleural fluid pH in loculated parapneumonic effusions. Chest 2004; 126: 2022-24
- Heffner J, Klein J. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 2008; 83: 235-50
- Bielsa S, Salud A, Martinez M et al. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Int Med 2008; 19: 334-36
- Sahn S, Good J. Pleural fluid pH in malignant effusions: diagnostic prognostic and therapeutic implications. Ann Intern Med 1988; 108: 345-49
- Rodriguez-Pandero F, López Mejias J. Survival time of patients with pleural metastatic carcinoma predicted by glucose and pH studies. Chest 1989; 95: 320-24
- Aelony Y, Yao J, King R. Prognostic value of pleural fluid pH in malignant epithelial mesothelioma after talc poudrage. Respiration 2006; 73: 334-39
- Rodriguez-Pandero F, Antony V. Pleurodesis: state of the art. Eur Respir J 1997; 10: 1648-54
- Antony V, Loddenkemper R, Astoul P et al. Management of malignant pleural effusions. Eur Respir J 2001; 18: 402-19
- Bowling M, Chatterjee A, Conforti J. Perceptions vs. reality: measuring of pleural fluid pH in North Carolina. NC Med J 2009; 70: 9-13
- Chandler T, McCoskey E, Byrd R et al. Comparison of the use and accuracy of methods for determining pleural fluid pH. South Med J 1999; 92: 214-17
- Kohn G, Hardie W. Measuring pleural fluid pH: high correlation of a handheld unit to a traditional tabletop blood gas analyzer. Chest 2000; 118: 1626-29
- ChengDS, Rodriguez R, Rogers J et al. Comparison of pleural fluid pH obtained using blood gas machine, pH meter, and pH indictor strip. Chest 1998; 144: 1368-72
- Lesh E, Roth B. Is pH paper an acceptable, low-cost alternative to the blood gas analyzer for determining pleural fluid pH? Chest 1997; 112: 1291-92
- Rahman N, Mishra E, Davies H et al. Clinically important factors influencing the diagnostic measurement of pleural fluid pH and glucose. Am J Respir Crit Care Med 2008; 178: 483-90
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









