Printed from acutecaretesting.org
June 2004
Accreditation of POCT facilities
Introduction
Over the past two decades the demand for point-of-care testing (POCT) facilities has increased. In part, this has been due to a perception that the central laboratory provides a poor service with respect to turnaround time (TAT). There is a requirement by clinicians to minimize TAT for certain tests [1] and this, together with improvements in POCT systems [2], has driven the demand for POCT.
Within intensive care units patients are frequently ventilated and it is accepted that blood gases, electrolytes and metabolites are performed by clinical staff using POCT systems [3].
In other critical care areas such as emergency departments there is an increasing need for rapidity of results driven by the clinical need for rapid diagnosis [4].
Additional factors can influence the introduction of POCT, such as reducing patient waiting times and admission rates [5]; for example, the UK target states that no patient should be waiting more than four hours in emergency departments from arrival to admission, transfer or discharge [6].
Along with increased usage of POCT in clinical areas, there has also been a rapid increase in the availability of home-testing POCT devices. It is now commonplace for diabetic patients to monitor their own blood glucose levels, and with an ever-ageing population it may be expected that there will be an increase in demand for these systems [7].
These factors have led to a rapid increase in the global market for POCT with worldwide costs increasing from USD 3 billion in 1997 to USD 5.4 billion by 2001 [8] and this is expected to double over the next decade [9].
Quality and accreditation in POCT
The concept of clinical governance within the healthcare sector as a framework by which quality improvement could be promoted, together with the introduction of accreditation standards for medical laboratories, has focused the debate towards the use of quality management systems as a mechanism by which quality may be continually improved.
There have been numerous sources of advice and guidance on quality improvement in the use of POCT, examples of some are provided below:
- 1995 Kost
Guidelines for point-of-care testing. Improving patient outcomes [10] - 1997 European Community Confederation of Clinical Chemistry (EC4)
Essential Criteria for Quality Systems in Medical Laboratories [11] - 1998 European Community Confederation of Clinical Chemistry (EC4)
Additional Essential Criteria for Quality Systems of Medical Laboratories [12] - 1999 The German Working Group on medical laboratory testing (AML)
Recommendations on the introduction and quality assurance of procedures for POCT in hospitals [13] - 2000 The UK Joint Working Group on Quality Assurance (JWGQA) [14]
Advice on the use of near-to-patient or point-of-care testing - 2002 The UK Medicines and Healthcare products Regulatory Agency (MHRA)
Recommendations regarding the selection of equipment and use of POCT [15]
Accreditation standards for medical laboratories and systems for assessing compliance vary from country to country. Some accrediting bodies make specific reference to POCT; for example, the College of American Pathologists (CAP) have produced a POCT checklist [16] based upon the Clinical Laboratory Improvement Act (CLIA ’88).
Within the UK, Clinical Pathology Accreditation (CPA) has defined ‘Standards for the Medical Laboratory’ [17].
Although these standards make no specific reference to POCT, there is an expectation that any laboratory-controlled POCT will conform to the general laboratory standards. Facilities outside a hospital, such as primary care offices/general practitioner's surgeries, are required in some countries to seek advice from the local hospital laboratory before embarking on POCT.
Within the UK, Standard 13 of the Primary Health Care Standards and Criteria of the King’s Fund Organisational Audit program states ‘Near patient testing conforms to protocols developed with an accredited pathology department’ [18].
Many medical laboratory accreditation standards now have a requirement for systems of quality management and continuous improvement; these include ISO 15189 and CPA UK. The focus on quality in the healthcare sector has increased the importance of accreditation of medical laboratories.
There can be little doubt that POCT will have to be included within the laboratory accreditation process and that in many countries this process will become mandatory [19].
Management of POCT
If laboratory staff are to successfully manage POCT and attain the standards required by accrediting bodies, they will need to gain the consensus and support of other health professionals. This may best be achieved by the formation of a POCT group made up of individuals with both the desire and expertise to address POCT issues.
If the group is to have credibility with other professionals, members should be drawn from varying professional backgrounds and different clinical environments and provide frontline experience of POCT and/or expert advice.
Once convened, the POCT group should identify the objectives and set down terms of reference. Objectives of a POCT group could include but may not be limited to:
- To define an organization-wide policy for the evaluation, selection and utilization of POCT devices
- To produce procedures detailing how this policy is implemented together with systems that would provide ongoing evidence of appropriate and correct implementation
- To understand the needs and requirements of POCT users, thus ensuring that systems are operated in a clinically effective manner
- To ensure the production of high-quality results whilst minimizing risk to patient and user
- To ensure that users are trained in use of POCT devices and that competence is tested and recorded
- To ensure integrity of data and maintain confidentiality according to national legislation
- To gain a clear understanding of the costs of POCT compared with central laboratory testing, ensuring efficiency of use and financial prudence.
Documentation
The backbone of all accreditation systems is the documentation on which the system is built and assessed.
Figure 1 serves to demonstrate how policy, procedures and records are linked into a quality system [20]. The policy provides a statement of intent of how POCT is to be performed within an organization and the quality standards that are to be achieved.
Procedures and instructions provide practical detail and information on how the policy is implemented.
Finally, forms and records provide unequivocal evidence of the correct implementation of the policy according to the procedures.
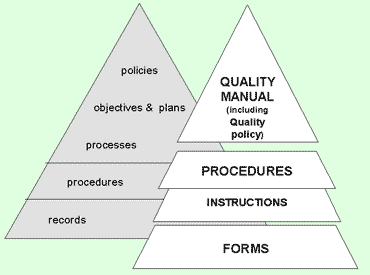
FIG. 1. Hierarchy of documentation From: Burnett D. A practical guide to accreditation. [20]
The POCT policy
The POCT policy serves as a high-level document that should consider areas impacting upon quality management in POCT.
Examples of possible areas to consider are provided in Table I.
The policy should be formally approved by top management within an organization and published in a medium that allows the widest possible access to potential users of POCT.
The policy should be reviewed and, where necessary, updated in accordance with a predefined schedule.
|
|
TABLE I. Main areas of POCT to consider
The POCT management procedure
The most significant procedure within any system is the management procedure that provides detail on how the system is operated.
The headings in Table II suggest possible content for a POCT management procedure. This provides detail on the process by which the policy would be implemented and the mechanisms that produce clear evidence of its implementation.
|
TABLE II. Content of a management procedure for POCT
Many models have been proposed for management of POCT and each has to be tailored to the particular institution. Although models are proposed from both laboratory and nursing standpoints, there is agreement that the designation of a person or persons as POCT coordinator(s) with substantial time allocated to the task will greatly enhance the success of any POCT activity.
In a large- or medium-size hospital, POCT activity in wards, clinics or other designated areas might be represented on the working group by an area supervisor. The management structure of POCT serves as the basis on which all aspects of POCT are delivered.
Other procedures
There will be a need for detailed procedures relating to all aspects of POCT. These may vary from the organization of training and certification of non-laboratory staff to detailed procedures on how POCT equipment is used or maintained.
It is important that all procedures are reviewed and updated on a regular basis and that they are signed off by an authorized individual. Many accreditation systems demand that procedures are controlled documents [17] and that where individuals hold personal copies there should be a mechanism for updating them to prevent risks associated with use of out-of-date material.
Some would argue that ownership of personal copies of a procedure or even notes should be actively discouraged or even prohibited.
Records and forms
In order to provide evidence of the correct implementation of a policy it is necessary to document all aspects of POCT.
Documents should be devised to enable an accurate record to be kept and stored for future reference, examples of such documents are provided in Table III. Forms and records may be used by external assessors from accrediting authorities in the same way and will allow the identification of areas of non-compliance with accreditation standards.
|
|
TABLE III. Examples of forms and records in POCT
Discussion
POCT is a rapidly developing area of diagnostic testing within the healthcare environment. In modern hospitals, POCT may account for up to 20 % of in vitro diagnostic tests performed.
There are significant risks from inappropriate use of POCT devices both in terms of potential harm to patients and unnecessary expenditure, where changes to laboratory testing protocols could resolve possible problems in the delivery of a service.
The formation of a group to manage POCT within the organization formalizes the process of introducing and monitoring POCT. As members of the POCT group are drawn from multidisciplinary, multiprofessional backgrounds they represent the interests and concerns of users, laboratory staff and significantly add to the credibility of the group.
The formalization of a POCT policy is essential to defining the standards by which POCT will be implemented and performed within the organization. Procedures provide important detail on how the test is implemented and forms provide evidence of appropriate implementation.
The combination of all of the above ensures that the quality of POCT within an organization is maintained at the highest level and formally reviewed on a regular basis.
The process of organizing and managing POCT within a single organization, such as a hospital, is relatively straightforward.
However, within the primary care system there are an ever-growing number of POCT devices. It is important that the local laboratory becomes actively involved in the provision of POCT with the primary healthcare sector.
References+ View more
- Azzazy HME, Christenson RH. Cardiac markers of acute coronary syndromes: is there a case for point-of-care testing? Clinical Biochemistry 2002; 35,1: 13-27.
- Crook MA. Near patient testing and pathology in the new millennium. J Clin Pathol 2000; 53,1: 27-30.
- Castro HJ, Oropello JM, Halpern N. Point-of-care testing in the intensive care unit. The intensive care physician's perspective. Am J Clin Pathol 1995; 104,4 Suppl 1: S95-S99.
- Storrow AB, Gibler WB. The role of cardiac markers in the emergency department. Clinica Chimica Acta 1999; 284,2: 187-96.
- Stubbs P, Collinson PO. Point-of-care testing: a cardiologist's view. Clinica Chimica Acta 2001; 311,1: 57-61.
- The NHS plan. A plan for investment. A plan for reform. London: The Stationery Office, 2000.
- Lehmann CA. The future of home testing--implications for traditional laboratories. Clinica Chimica Acta 2002; 323,1-2: 31-36.
- Freedman DB. Clinical governance: implications for point-of-care testing. Ann Clin Biochem 2002; 39,Pt 5: 421-23.
- Bissell M, Sanfilippo F. Empowering patients with point-of-care testing. Trends Biotechnol 2002; 20,6: 269-70.
- Kost GJ. Guidelines for point-of-care testing. Improving patient outcomes. Am J Clin Pathol 1995; 104,4 Suppl 1: S111-27.
- Jansen RT, Blaton V, Burnett D, Huisman W, Queralto JM, Zerah S. Essential criteria for quality systems in medical laboratories. European Community Confederation of Clinical Chemistry (EC4) Working Group on Harmonisation of Quality Systems and Accreditation. Eur J Clin Chem Clin Biochem 1997; 35,2: 123-32.
- Jansen RT, Blaton V, Burnett D, Huisman W, Queralto JM, Zerah S et al. Additional essential criteria for quality systems of medical laboratories. European Community Confederation of Clinical Chemistry (EC4) Working Group on Harmonisation of Quality Systems and Accreditation. Clin Chem Lab Med 1998; 36,4: 249-52.
- Briedigkeit L, Muller-Plathe O, Schlebusch H, Ziems J. Recommendations of the German Working Group on medical laboratory testing (AML) on the introduction and quality assurance of procedures for point-of-care testing (POCT) in hospitals. Clin Chem Lab Med 1999; 37,9: 919-25.
- Near to patient or point of care testing. The Joint Working Group on Quality Assurance. Clin Lab Haematol 2000; 22,3: 185-88.
- Medical Devices Agency. Management and use of IVD point of care test devices. MDA, 2002.
- College of American Pathologists Commission on Laboratory Accreditation. Point of care testing checklist. College of American Pathologists, 2001.
- Standards for the Medical Laboratory. Clinical Pathology Accreditation (UK) Ltd. Website [1.0]. 2001. CPA UK Ltd.
- King's Fund Organisational Audit. Primary health care standards and criteria. London: King's Fund, London 1993.
- Burnett D. Accreditation and point-of-care testing. Ann Clin Biochem 2000; 37, Pt 3: 241-43.
- Burnett D. A practical guide to accreditation. London: ACB Venture Publications, 2002.
References
- Azzazy HME, Christenson RH. Cardiac markers of acute coronary syndromes: is there a case for point-of-care testing? Clinical Biochemistry 2002; 35,1: 13-27.
- Crook MA. Near patient testing and pathology in the new millennium. J Clin Pathol 2000; 53,1: 27-30.
- Castro HJ, Oropello JM, Halpern N. Point-of-care testing in the intensive care unit. The intensive care physician's perspective. Am J Clin Pathol 1995; 104,4 Suppl 1: S95-S99.
- Storrow AB, Gibler WB. The role of cardiac markers in the emergency department. Clinica Chimica Acta 1999; 284,2: 187-96.
- Stubbs P, Collinson PO. Point-of-care testing: a cardiologist's view. Clinica Chimica Acta 2001; 311,1: 57-61.
- The NHS plan. A plan for investment. A plan for reform. London: The Stationery Office, 2000.
- Lehmann CA. The future of home testing--implications for traditional laboratories. Clinica Chimica Acta 2002; 323,1-2: 31-36.
- Freedman DB. Clinical governance: implications for point-of-care testing. Ann Clin Biochem 2002; 39,Pt 5: 421-23.
- Bissell M, Sanfilippo F. Empowering patients with point-of-care testing. Trends Biotechnol 2002; 20,6: 269-70.
- Kost GJ. Guidelines for point-of-care testing. Improving patient outcomes. Am J Clin Pathol 1995; 104,4 Suppl 1: S111-27.
- Jansen RT, Blaton V, Burnett D, Huisman W, Queralto JM, Zerah S. Essential criteria for quality systems in medical laboratories. European Community Confederation of Clinical Chemistry (EC4) Working Group on Harmonisation of Quality Systems and Accreditation. Eur J Clin Chem Clin Biochem 1997; 35,2: 123-32.
- Jansen RT, Blaton V, Burnett D, Huisman W, Queralto JM, Zerah S et al. Additional essential criteria for quality systems of medical laboratories. European Community Confederation of Clinical Chemistry (EC4) Working Group on Harmonisation of Quality Systems and Accreditation. Clin Chem Lab Med 1998; 36,4: 249-52.
- Briedigkeit L, Muller-Plathe O, Schlebusch H, Ziems J. Recommendations of the German Working Group on medical laboratory testing (AML) on the introduction and quality assurance of procedures for point-of-care testing (POCT) in hospitals. Clin Chem Lab Med 1999; 37,9: 919-25.
- Near to patient or point of care testing. The Joint Working Group on Quality Assurance. Clin Lab Haematol 2000; 22,3: 185-88.
- Medical Devices Agency. Management and use of IVD point of care test devices. MDA, 2002.
- College of American Pathologists Commission on Laboratory Accreditation. Point of care testing checklist. College of American Pathologists, 2001.
- Standards for the Medical Laboratory. Clinical Pathology Accreditation (UK) Ltd. Website [1.0]. 2001. CPA UK Ltd.
- King's Fund Organisational Audit. Primary health care standards and criteria. London: King's Fund, London 1993.
- Burnett D. Accreditation and point-of-care testing. Ann Clin Biochem 2000; 37, Pt 3: 241-43.
- Burnett D. A practical guide to accreditation. London: ACB Venture Publications, 2002.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








