Printed from acutecaretesting.org
February 2020
All you need to know about troponin measurements
Introduction
This article features a general background of the analytical characteristics associated with cardiac troponin testing.
It addresses the following areas:
- Introduction to troponin
- What is the 99th percentile upper reference limit (URL) and why do we use it?
- Determination of the 99th percentile URL
- The dynamics of troponin, and the need for serial measurements
- All about sensitivity and precision
- All about change, when to draw and measure the 2nd sample
- Implications of assay performance on rule-in and rule-out algorithms for acute myocardial infarction (MI)
- Summary
Introduction to troponin
Troponin is one of the regulatory proteins in muscle tissue. The heart muscle, or myocardium, contains a cardiac-specific type of troponin (cTn). In case of myocardial injury and cellular damage, cTn is released from the myocardium into the blood. Specific monoclonal antibodies against cTn have enabled the development of cTn-specific assays that can measure the concentration of cTn in the blood. Since the first publications on cardiac troponin (cTn) in the 1970s, around 20.000 scientific reports on this powerful biomarker of myocardial injury have been published. Over time, cTn assays have improved their analytical performance and become the designated laboratory biomarker for diagnosing myocardial injury and myocardial infarction (MI). Finally, the larger the myocardial insult, the more troponin is released into circulation. Consequently, quantitative cTn results also provide prognostic value in support of risk stratification and patient management [1,2,3].
What is the 99th percentile URL and why do we use it?
Because cTn is not a constitutive blood protein, blood levels of cTn will be extremely low in healthy individuals without myocardial damage. In contrast to many other blood biomarkers that can show abnormally low and abnormally high levels, the clinical interest in cTn levels pertains therefore to increased levels only.
The first generations of cTn-assays were, not surprisingly, hardly able to report quantitative cTn results in individuals NOT having myocardial injury. In most of these cases, a “lower than level of detection” (< LoD) result was reported. Even today, many of the contemporary cTn assays report measurable cTn levels in just a few percent of healthy controls.
To some degree, this also explains the origin of using the 99th percentile URL as the cut-off between normal and increased cTn levels. When the usual 97.5th percentile cut-off would have been chosen, e.g. as applied for most biomarkers, many contemporary cTn assays would have struggled to generate more than 2.5% measurable results required for establishing a 97.5% cut-off based on non-parametric statistics [4].
The most important reason for using the 99th percentile URL however is the current lack of harmonization between, and standardization of, the various cTn assays. This prohibits the application of one numeric cut-off across all cTn assays as a physiological threshold for myocardial injury [5,6]. Instead, the 99th percentile URL provides a mandatory “stick-in-the-ground” for every cTn assay and the analytical cut-off between normal and increased cTn levels. The clinical importance of the 99th percentile URL is therefore clear, and the continuous interest of experts in cTn assays’ 99th percentile URL a logical consequence.
Consequently, cTn assay manufacturers are required to provide their assay-specific 99th percentile URL in the IFU. However, this value will be typically based on a regional population of healthy controls, which might not provide the optimal value for all users across the globe. Factors like age, gender, and ethnicity can and will affect cTn levels and significantly influence the URL. A careful local validation of this IFU-based 99th percentile URL is therefore advisable [7]. Moreover, in case any local population bias is anticipated, local derivation of the 99th percentile URL cut-off is recommendable, as will be discussed later.
Aside the 99th percentile URL cut-off, the precision of the assay at that level is also important, as we will discuss later.
Determination (and monitoring) of the 99th percentile URL
The 99th percentile URL for a specific assay is calculated from the results obtained in a healthy control population. According to guidance [7], for qualified determination of the 99th percentile URL for a contemporary cTn assay, a population of at least 300 healthy individuals is required with an appropriate age, ethnic and gender mix. In the case of high-sensitivity assays, the 99th percentiles are gender-specific, and hence, at least 300 healthy females and 300 healthy males should be tested [7]. For contemporary assays, with most of the results
Several co-morbidities and/or confounders are known to increase the circulating level of cTn [9,2]. When some of the results used to determine the 99th percentile URL are affected by such co-morbidities, the 99th percentile URL can easily be affected as well. Consequently, all subjects enrolled in the healthy reference population need to be pre-screened to reduce to potential influence of such factors. The importance of such thorough pre-selection in determination of the appropriate 99th percentile URL of cTn assays has been shown in many studies [10,9,11]. Overall, it is recommended that selection of individuals for healthy reference populations should at least include a health questionnaire and several laboratory tests to ensure normal renal function and absence of congestive heart failure.
Many studies have reported alternative cut-offs for the various cTn assays, deviating from the 99th percentile URL as provided by the manufacturer in the IFU, and often associated with superior diagnostic performance [12,13,14,15]. Current best practices, based on using the 99th percentile URL with cTn assays to achieve optimal diagnostic accuracy for myocardial injury, should therefore include critical monitoring of the cTn-assay in routine use with respect to calibration and verification of precision, especially at the diagnostic cut-off [16,2].
Over time, various generations of troponin assays have been developed with ever-increasing sensitivity and ever lower 99th percentiles. Consequently, as illustrated in Figure 1, the newer generations of cTn assays can detect increased cTn levels earlier, associated with increased sensitivity for myocardial injury and faster rule-in and rule-out of ACS. However, the latest generations of high-sensitivity (hs-cTn) assays also caused a lot of discussion and even controversy regarding their optimal use in routine clinical diagnostics. Especially the increased risk for over-diagnosis of MI [2] and the increasing complexity associated with the use of hs-cTn assays have raised some general concerns [2,17].

Figure 1. The evolution of cTn assay sensitivity and 99th percentiles URL.
The dynamics of troponin and the need for serial measurements
After an acute cardiac insult and a brief period of local ischemia, myocardial cells are starting to die (necrosis) and cardiac troponin is released. Typically, during the first several hours after the insult, patients start feeling a blunt pain in/on their chest and/or in their arms. Obviously, they will seek urgent medical attention and in most cases be admitted to a chest pain unit or emergency department (ED) of a nearby hospital. Depending on the type of MI, cTn levels reach their maximum between 6 hours and 3 days, and then start to decline [18]. Normally, cTn levels require several weeks to return to baseline, provided that no incremental myocardial damage is inflicted during that time.
According to the current definition of MI and the current clinical guidelines, acute MI is diagnosed in the presence of acute myocardial injury, evident by an increased and significant rise and/or fall of cardiac troponin (cTn) levels, in combination with clinical signs of myocardial ischemia, such as pathological ECG changes [1,6].
A positive cTn result supporting the diagnosis of acute MI requires therefore:
- At least one sample with increased cTn, e.g. exceeding the 99th percentile URL
- A significant change in cTn levels, e.g. “delta”, between two serial measurements
The following chapters will focus on both requirements for the various categories of cTn assays in more detail.
All about sensitivity and precision
The detection limits of a cTn assay depend on its sensitivity and precision. Higher sensitivity and precision result in earlier detection of increased or changed cTn concentrations, and hence, earlier detection of acute myocardial injury and MI. Current guidelines recommend using assays with a total CV ≤ 10% at the 99th percentile URL, classifying such assays as “guideline acceptable” [19]. Acceptable in clinical routine are also cTn assays with a total CV at the 99th percentile URL between 10% and 20%, making such assays “clinically usable” because the risk of misclassification patients using these assays is low [19].
With the further development of cTn assays, the 99th percentiles URL of the assays get ever lower, and the sensitivity gets ever higher. The latest generation of hs-cTn assays is not only able to offer a CV ≤ 10% at the 99th percentile URL but can also measure cTn concentrations above the level of detection (LoD) in more than 50% of a healthy population. Moreover, hs-cTn assays have uncovered gender-specific 99th percentiles URL, the value for females typically found lower than for males (Figure 2).
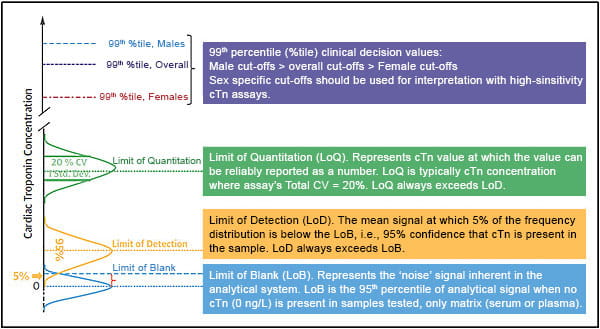
An understanding of these terms facilitates transition to high-sensitivity cardiac troponin testing. %tile = percentile; cTn = cardiac troponin; CV = coefficient of variation; LoB = Limit of Blank; LoD = Limit of Detection; LoQ = Limit of Quantitation; Std. Dev. = standard deviation.
Figure 2. Various Analytic Definitions to Familiarize Clinicians (with permission from Journal of the American College of Cardiology [2].
Because of the ever-higher sensitivities of cTn assays, ever-lower levels of myocardial injury are detected. Although such minor injury can be caused by a small size infarction, more often the etiology behind it is not ischemic injury, but rather one of the many other causes of myocardial injury, including chronic disease [20,21,22,17]. The high sensitivity of hs-cTn assays can therefore cause confusion in the diagnostic work-up of chest pain patients, especially when positive cTn results are not confirmed by positive ECG results [21,22,2].
Aside the sensitivity, which depends primarily on the 99th percentile, also the precision of the assay plays an important role in the diagnosis of acute myocardial injury. Newer generations of cTn assays typically have higher sensitivity as well as higher precision (or lower imprecision). As mentioned earlier, the total imprecision at the 99th percentile URL classifies the assay as guideline acceptable (CV ≤ 10 %) or clinically usable (CV > 10 % and ≤ 20 %).
The better the precision is, the higher the probability that a found difference between two serial samples is significant and indicative of acute myocardial injury.
All about change, when to draw and measure the 2nd sample?
In clinical practice, a significant change in cTn levels between two serial samples will be detectable earlier with a “guideline acceptable” assay than with “clinically usable” assay, and with an hs-cTn assay even earlier. In other words, for detecting acute myocardial injury with a “clinically usable” assay one will need more time between the serial tests compared to a “guideline acceptable” test. To appreciate the difference, please refer to Figure 3 where the difference between the two classes of cTn assays on detecting a significant change in cTn levels (“delta”) is illustrated. In this model, we have assumed that the 99th percentile of both assays is the same, illustrated by the dotted blue line.
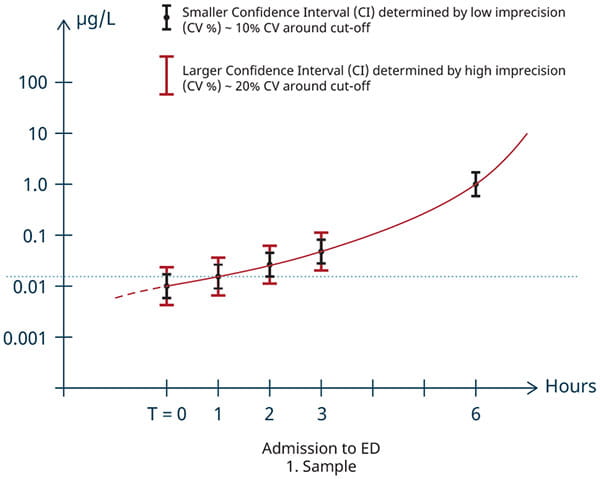
Figure 3. Illustration of the diagnostic performance of “guideline acceptable” and “clinical usable” troponin assays
To diagnose acute myocardial injury, it is essential to demonstrate a significant change “delta” in the level of cTn between two serial cTn results with at least one result above the 99th percentile URL. When both cTn results from two serial samples are found above the 99th percentile URL, then such a “delta”, most preferable a pre-defined assay-specific absolute change [20], is often larger and easier to be detected. This change is indicative of an ongoing acute pathology [1,6]. As shown in Figure 3, an assay with lower imprecision will be able to detect such significant change in cTn levels earlier and require a shorter interval between serial measurements.
Figure 3 also illustrates that a decrease in the cut-off would lead to faster rule-in for both contemporary and high sensitivity assays, and why it is recommended for users of troponin assays to determine a cut-off based on their local population, especially when local population bias is anticipated [7]. For example, in clinics treating predominantly a sub-population of patients, e.g. young, old, renal disease patients, or a particular ethnic group.
Based on current literature and best practices, a time interval of 6-12 hours between sample draws for serial cTn testing seems to provide optimal diagnostic accuracy for most currently used contemporary cTn assays. More sensitive assays, including the high-sensitivity assays, can provide adequate diagnostic performance within 3-6 hours, or even faster in selected patients or by applying very rigorous criteria [1,6,23].
Irrespective of the cTn assay used in local clinical practice, in general it is recommended to develop a standardized serial sampling protocol in clinical institutions for patients admitted with suspected ACS, appropriate for their local cTn assay [10,2]. The protocol should include the thresholds for significant change, indicative of acute myocardial injury.
Implications of assay performance on rule-in and rule-out algorithms for acute MI
Detecting increased and changing levels of cTn, in combination with clinical signs of ischemia, will support the diagnosis of acute MI and referral of the patient to further diagnostic work-up and/or invasive management.
When using a contemporary assay, myocardial injury will be diagnosed by a cTn measurement at entry of ED and repeating the cTn measurement after the appropriate sample interval of about 6 hours, dependent on the assay used.
When using a high-sensitivity assay, high cTn levels at entry of ED (>5 x 99th percentile URL) and positive ECG changes can help to refer patients to immediate invasive treatment. Normal cTn levels (<99th percentile URL), in combination with normal ECG, will support earlier rule-out of MI and discharge of such a patient [23]. Especially in case of late arrivals (chest pain > 6 hours prior to admission) negative cTn results will support exclusion of MI. Negative cTn results in early arrivals, or moderately positive hs-cTn results, will require retesting at 3-6 hours [24].
The upside of hs-cTn testing is clearly related to its high sensitivity. High sensitivity results in low risk of missing out on patients having an acute MI. High sensitivity also supports early and safe rule-out algorithms, even when a lower percentage of patients might benefit when compared to the rule-out performance offered by contemporary cTn assays [22,25,26].
The downside of the increased sensitivity of the hs-cTn assays is the decreased specificity and increased risk for false positive results suggesting ACS. Although the risk for missing MI is smaller when using an hs-cTn assay, especially elderly patients without ACS, or patients with other morbidities such as renal disease might be showing moderately increased and/or changed levels of cTn. This increased level of false positives associated with hs-cTn testing will result in an increased need for diagnostic follow-up, and a risk for inappropriate treatment [25,26].
Medical facilities and clinical staff are trying to avoid such unjustified follow-up, for both logistical and economic reasons. The additional costs and care associated with unjustified diagnosis of MI associated with the lower specificity of hs-cTn testing is therefore a growing concern [25,26]. Moreover, implementation of a hs-cTn testing is associated with several practical requirements to ensure its appropriate diagnostic and clinical use [2]. Despite these concerns, the introduction of hs-cTn assays will further enhance the early diagnosis and safe rule-out of myocardial infarction and therefore presents an essential biomarker for the clinical lab in the diagnostic work-up of patients with suspected ACS.
Summary
- Increased and changing levels of cTn are key in the clinical diagnosis of acute MI.
- Such level of diagnostic performance can be provided by cTn assays demonstrating an imprecision (CV) of 20% or better at the diagnostic cut-off.
- The diagnostic cut-off of every cTn assay is the 99th percentile URL as determined in a population of healthy controls for that assay.
- A risk of population bias exists in the patient population tested by the user, as well as in the control population used by the manufacturer to determine the assay’s cut-off.
- Local validation or derivation of the 99th percentile URL is recommendable, especially when population bias is anticipated.
- Lower cut-offs and lower imprecision of cTn assays are typically associated with increasing sensitivity of the assay.
- With increasing sensitivity and precision of cTn assays, diagnosis and rule-out of acute MI can be achieved earlier and faster.
References+ View more
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation 2018; 138:618-51.
- Januzzi JL, Mahler SA, Christenson RH, et al. Recommendations for institutions transitioning to high-sensitivity troponin testing - JACC scientific expert panel. JACC 2019; 73:1059–77.
- deFilippi CR, Herzog CA. Interpreting Cardiac Biomarkers in the Setting of Chronic Kidney Disease. Clin Chem. 2017; 63:59-65.
- Apple FS, Ler R, and Murakami MM. Determination of 19 Cardiac Troponin I and T Assay 99th Percentile Values from a Common Presumably Healthy Population. Clin Chem. 2012; 58:1574–81.
- Wildi K, Gimenez MR, Twerenbold R, et al. Misdiagnosis of myocardial infarction related to limitations of the current regulatory approach to define clinical decision values for cardiac troponin. Circulation 2015; 131:2032–40.
- Apple FS, Collinson PO; IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012; 58:54–61.
- Wu AHB, Christenson RH, Greene DN, et al. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: Expert opinion from the Academy of the American Association for Clinical Chemistry and the task force on clinical applications of cardiac biomarkers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018; 64:645–655.
- Eggers KM, Apple FS, Lind L, and Lindahl B. The applied statistical approach highly influences the 99th percentile of cardiac troponin I. Clin Biochem 2016; 49:1109-12.
- Sandoval Y, Apple FA. The global need to define normality: The 99th percentile value of cardiac troponin. Clin Chem. 2014; 60:3455–62.
- Collinson PO, Heung YM, Gaze D, et al. Influence of Population Selection on the 99th Percentile Reference Value for Cardiac Troponin Assays. Clin Chem. 2012; 58:219-25.
- Wilke P, Masuch A, Fahron O, et al. Diagnostic performance of point-of-care and central laboratory cardiac troponin assays in an emergency department. PLoS ONE 2017; 12(11): e0188706.
- Mion, M. M, Bragato G, Casarotti A, et al. Clinical performance of cardiac Troponin I: A comparison between the POCT AQT90 FLEX and the Dimension Vista analyzer in an emergency setting. Clin Biochem. 2017; 50:763-67.
- Ivandic BT, Spanuth E, Giannitsis E. Performance of the AQT90 FLEX cTnI point-of-care assay for the rapid diagnosis of acute myocardial infarction in the emergency room. Clin Lab. 2014; 60:903-8.
- Greiser A, Winter T, Mahfoud H, et al. The 99th percentile and imprecision of point-of-care cardiac troponin I in comparison to central laboratory tests in a large reference population. Clin Biochem. 2017; 50:1198-202.
- Suh D, Keller DI, Hof D, et al. Rule-out of non-ST elevation myocardial infarction by five point of care cardiac troponin assays according to the 0 h/3 h algorithm of the European Society of Cardiology. Clin Chem Lab Med. 2018; 56:649-57.
- Herman DS, Kavsak PA, Greene DN. Variability and Error in Cardiac Troponin Testing: An ACLPS Critical Review. Am J Clin Path. 2017; 148:281-95.
- De Hann Jacob. Preparing for high sensitivity troponin testing. Acutecaretesting.org. Dec. 2019.
- Van Beek D, van Zaane B, Looije M, et al. Typical rise and fall of troponin in (peri-procedural) myocardial infarction: A systematic review. World J Cardiol 2016; 8:293-301.
- Apple FS. A New Season for Cardiac Troponin Assays; It’s time to keep a scorecard. Clin Chem. 2009; 55:1303-306.
- Sandoval Y, Smith SW, Thordsen SE, et al. Diagnostic performance of high sensitivity compared with contemporary cardiac troponin I for the diagnosis of acute myocardial infarction. Clinical Chemistry 2017; 63:1594-604.
- Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res 2017; 113:1708-18.
- Lee KK, Noaman A, Vaswani A, et al. Prevalence, determinants, and clinical associations of high-sensitivity cardiac troponin in patients attending emergency departments. Am J of Med. 2019; 132:9−22.
- Cullen LA, Mills NL, Mahler S, and Body R. Early rule-out and rule-in strategies for myocardial infarction. Clin Chem 2017; 63:129-39.
- Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal 2016; 37:267–315.
- Vasile VS, Jaffe AS. High-Sensitivity Cardiac Troponin for the Diagnosis of Patients with Acute Coronary Syndromes. Curr Cardiol Rep. 2017; 19:92.
- Fitzgerald G, Kerley RN, and Kiernan TJ. High-sensitivity troponin assays: development and utility in a modern health-care system. Expert Rev Cardiovasc Ther 2019; 17:763-70.
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation 2018; 138:618-51.
- Januzzi JL, Mahler SA, Christenson RH, et al. Recommendations for institutions transitioning to high-sensitivity troponin testing - JACC scientific expert panel. JACC 2019; 73:1059–77.
- deFilippi CR, Herzog CA. Interpreting Cardiac Biomarkers in the Setting of Chronic Kidney Disease. Clin Chem. 2017; 63:59-65.
- Apple FS, Ler R, and Murakami MM. Determination of 19 Cardiac Troponin I and T Assay 99th Percentile Values from a Common Presumably Healthy Population. Clin Chem. 2012; 58:1574–81.
- Wildi K, Gimenez MR, Twerenbold R, et al. Misdiagnosis of myocardial infarction related to limitations of the current regulatory approach to define clinical decision values for cardiac troponin. Circulation 2015; 131:2032–40.
- Apple FS, Collinson PO; IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012; 58:54–61.
- Wu AHB, Christenson RH, Greene DN, et al. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: Expert opinion from the Academy of the American Association for Clinical Chemistry and the task force on clinical applications of cardiac biomarkers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018; 64:645–655.
- Eggers KM, Apple FS, Lind L, and Lindahl B. The applied statistical approach highly influences the 99th percentile of cardiac troponin I. Clin Biochem 2016; 49:1109-12.
- Sandoval Y, Apple FA. The global need to define normality: The 99th percentile value of cardiac troponin. Clin Chem. 2014; 60:3455–62.
- Collinson PO, Heung YM, Gaze D, et al. Influence of Population Selection on the 99th Percentile Reference Value for Cardiac Troponin Assays. Clin Chem. 2012; 58:219-25.
- Wilke P, Masuch A, Fahron O, et al. Diagnostic performance of point-of-care and central laboratory cardiac troponin assays in an emergency department. PLoS ONE 2017; 12(11): e0188706.
- Mion, M. M, Bragato G, Casarotti A, et al. Clinical performance of cardiac Troponin I: A comparison between the POCT AQT90 FLEX and the Dimension Vista analyzer in an emergency setting. Clin Biochem. 2017; 50:763-67.
- Ivandic BT, Spanuth E, Giannitsis E. Performance of the AQT90 FLEX cTnI point-of-care assay for the rapid diagnosis of acute myocardial infarction in the emergency room. Clin Lab. 2014; 60:903-8.
- Greiser A, Winter T, Mahfoud H, et al. The 99th percentile and imprecision of point-of-care cardiac troponin I in comparison to central laboratory tests in a large reference population. Clin Biochem. 2017; 50:1198-202.
- Suh D, Keller DI, Hof D, et al. Rule-out of non-ST elevation myocardial infarction by five point of care cardiac troponin assays according to the 0 h/3 h algorithm of the European Society of Cardiology. Clin Chem Lab Med. 2018; 56:649-57.
- Herman DS, Kavsak PA, Greene DN. Variability and Error in Cardiac Troponin Testing: An ACLPS Critical Review. Am J Clin Path. 2017; 148:281-95.
- De Hann Jacob. Preparing for high sensitivity troponin testing. Acutecaretesting.org. Dec. 2019.
- Van Beek D, van Zaane B, Looije M, et al. Typical rise and fall of troponin in (peri-procedural) myocardial infarction: A systematic review. World J Cardiol 2016; 8:293-301.
- Apple FS. A New Season for Cardiac Troponin Assays; It’s time to keep a scorecard. Clin Chem. 2009; 55:1303-306.
- Sandoval Y, Smith SW, Thordsen SE, et al. Diagnostic performance of high sensitivity compared with contemporary cardiac troponin I for the diagnosis of acute myocardial infarction. Clinical Chemistry 2017; 63:1594-604.
- Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res 2017; 113:1708-18.
- Lee KK, Noaman A, Vaswani A, et al. Prevalence, determinants, and clinical associations of high-sensitivity cardiac troponin in patients attending emergency departments. Am J of Med. 2019; 132:9−22.
- Cullen LA, Mills NL, Mahler S, and Body R. Early rule-out and rule-in strategies for myocardial infarction. Clin Chem 2017; 63:129-39.
- Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal 2016; 37:267–315.
- Vasile VS, Jaffe AS. High-Sensitivity Cardiac Troponin for the Diagnosis of Patients with Acute Coronary Syndromes. Curr Cardiol Rep. 2017; 19:92.
- Fitzgerald G, Kerley RN, and Kiernan TJ. High-sensitivity troponin assays: development and utility in a modern health-care system. Expert Rev Cardiovasc Ther 2019; 17:763-70.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars







