Printed from acutecaretesting.org
December 1998
An evidence-based approach to hyperbaric wound healing
Wound healing management algorithms increasingly incorporate hyperbaric oxygen (HBO) therapy, and the premise for its use is sound.
Oxygen plays key nutritional and “cell signal” roles across the many phases of wound healing: hypoxia is a common etiology in wounds that fail to respond to standard management within anticipated time lines, and hyperbaric doses of oxygen are known to increase hypoxic wound oxygen tensions to normal, even supraphysiologic levels. If HBO therapy is to be effective, therefore, prospective demonstration of hypoxia is critical.
Equally important is the determination of a therapeutic end point if this esoteric and expensive intervention is to be cost-effectively applied.
Historically, these two issues were largely overlooked, resulting in extensive courses of therapy that all too frequently involved poor clinical outcomes at great expense to the health insurance system.
The modern application of HBO therapy in problem wound healing has evolved along “evidence-based” algorithmic lines. Transcutaneous oxygen technology is incorporated throughout the consultation and case management process. Periwound transcutaneous oxygen mapping is employed to:
1) identify the presence of underlying hypoxia, 2) assess whether regional perfusion is present in sufficient volumes to transfer centrally delivered hyperbaric oxygenation to the wound margin, 3) test for early angiogenic response, and 4) evaluate for “normalized” tissue transcutaneous oxygen/patient host competency.
The net results are more appropriate patient selection, and therapeutic dosing limited to “normalizing” the wound healing process rather than treating hyperbarically to complete wound resolution. In this locally host-competent state spontaneous healing continues with conservative wound care alone.Such brief courses of algorithmically applied HBO therapy greatly improve clinical outcomes while markedly reducing expenditures.
INTRODUCTION
Over the past decade the North American practice of hyperbaric medicine has been dominated by the problem wound referral. While this has not yet reached the same extent elsewhere, it may soon do so. There is growing appreciation of the therapeutic impact of hyperbaric oxygen therapy in the management of wound healing complications [1,2,3].
The premise on which hyperbaric doses of oxygen are prescribed is correction of hypoxia. A number of factors are known to compromise the orderly process of wound repair [4], but hypoxia is dominant among them [5]. Hypoxia serves to impair healing [6] and weaken host defenses [7].
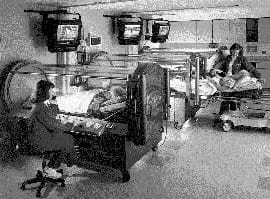 |
FIG 1: Single-occupancy hyperbaric chambers. |
Traditional thinking is that oxygen is simply a metabolite. Healing, therefore, is considered to be dependent upon the availability of adequate volumes of oxygen. Recent research, however, suggests that oxygen’s role is far more complex [8].
While clearly serving a vital nutritional function, oxygen appears to represent a key cell signal or “growth factor” [9,10]. Tissues otherwise rendered hypoxic respond to hyperbarically induced supraphysiologic elevations in oxygen in sufficient degrees to stimulate normal wound healing responses. Daily, such exposures may act to initiate and subsequently reinforce oxygen-dependent mechanisms that regulate the control of wound healing.
Where tissue hypoxia is large-vessel mediated, work-up for medical or surgical flow augmentation takes precedence. There is no point in employing hyperbaric oxygenation in patients who lack the physiologic capacity to respond locally (the wound) to centrally (the lung) delivered hyperbaric hyperoxia.
The benefits of hyperbaric treatment are derived from its systemic delivery [11]. There is no topical effect of oxygen on cutaneous wound healing [12]. Inspired oxygen must be capable of travelling from the lungs via the heart to the central then peripheral vasculature, in order to arrive at the wound margin.
Unless the distinction regarding relative contributions of large- and small-vessel pathology is made, it is difficult to justify hyperbaric oxygen therapy on both clinical and economic grounds. One only has to review the early application of hyperbaric medicine for problem wounds to appreciate this point.
It was common for patients to undergo very extensive courses of hyperbaric oxygen therapy, exceeding 100 treatments in some cases. Invariably, no attempt was made to determine whether or not reversible hypoxia was complicating wound healing. As one might expect, clinical outcomes were mixed, and financial expenditures poorly justified.
The modern day application of hyperbaric oxygen therapy is infinitely more discriminating. Today’s goal is not to employ hyperbaric oxygen therapy to heal the wound, per se. Rather, it is to “normalize” the environment around the wound by producing a critical mass of angiogenesis [13].
The patient is presumably converted to a locally host-competent state, and able to support spontaneous healing, from an oxygen-dependent standpoint. This management approach has considerable clinical and financial implications. Applied appropriately, it will elevate hyperbaric oxygen therapy to the position of credibility and respect it should have enjoyed several decades ago.
HYPERBARIC OXYGEN THERAPY
Hyperbaric oxygen is a treatment in which patients breath 100 % oxygen at greater than sea level pressure (1.0 atmosphere absolute). High pressures are achieved by the use of single- (Fig. 1) or multiple-occupancy hyperbaric chambers. Pressures range from 2.0 to 3.0 atmospheres absolute (200-300 % oxygen equivalent), and treatments last for approximately 90 minutes. A course of treatments will range from 1 to 50, depending on the condition to be treated.
Hyperbaric medicine is by no means new. It has been employed since the late 1800s. Until the second half of the 20th century, however, its application was limited to the treatment of divers and others who suffered decompression accidents. Its beneficial mechanism was based upon the simple concept of Boyle’s Law.
If pressure is increased (such as in a hyperbaric chamber), gas volume (bubbles within the bloodstream, i.e., decompression sickness or “the bends”) decreases. Today, decompression illness represents a mere fraction of the total number of cases referred to hyperbaric medicine programs.
|
TABLE I
|
The modern application of this unique medical technology is infinitely more sophisticated. Intermittent exposure to hyperbaric doses of oxygen has been demonstrated to produce a number of complex physiological and biochemical effects. In turn, these effects represent a series of “beneficial mechanisms” (Table I).
A great deal of published research, involving many thousands of articles, has addressed these mechanisms and the various conditions that might benefit from them. The majority of this work has been conducted at the basic science level. It is only quite recently that controlled human data have begun to validate clinical efficacy [20,21] and cost effectiveness [22,23].
Today, in carefully selected and algorithmically managed patients, hyperbaric oxygen therapy:
- Confers advanced wound healing in otherwise refractory and chronic ischemic lesions [2,3] with enduring results [23];
- Markedly reduces the incidence of amputation in both diabetic patients [20,23] and trauma victims [21];
- Is central nervous system sparing [25];
- Decreases morbidity and mortality in certain anaerobic [26] and mixed soft-tissue infections [27];
- Reduces length of stay and need for skin grafting in acute thermal burns [28].
|
TABLE II
|
The North American practice of hyperbaric medicine is based upon an Undersea and Hyperbaric Medical Society listing of “Approved Uses” [11] (Table II). It will be noted that some of these conditions are uncommon. Further, many involve more established procedures and therapies initially, with hyperbaric oxygen reserved for refractory or more complex cases.
The evaluation and management of refractory wound healing and related financial expenditures, however, represent an enormous burden on the healthcare system. In one study, 16 % of all hospital admissions and 23 % of total hospital days were the result of diabetic foot lesions [29]. All too frequently, the care of these patients is unsuccessful. It has been estimated that several billion dollars is expended in the United States each year to amputate the extremities and rehabilitate diabetics suffering foot and leg lesions [23].
To fully appreciate the enormity of the “wound healing problem”, one must also consider the many other etiologies known to complicate wounds and retard their healing. Peripheral arterial occlusive disease, venous stasis disease, late radiation tissue injury, and sickle cell disease, are examples.
Not surprisingly, “problem wound healing” patients represent a significant percentage of the total hyperbaric referral volume.
TRANSCUTANEOUS pO2 MEASUREMENTS
The critical role of oxygen in wound healing has been the subject of several decades of research and has been extensively reviewed elsewhere [2,6]. Some local wound hypoxia is inevitable in injured tissue states and is thought to act as a stimulus for repair [6]. Local ischemia, however, is a completely different matter.
Flow deficits and resultant reductions in oxygen delivery represent a major impediment to healing [6]. Therefore, to effectively manage hypoxic wounds one must work up for ischemia. Findings of ischemia are commonly the result of macro- and/or microvascular disruptions. Distinction between large- and small-vessel disease is important.
If ischemia is significant and large-vessel mediated, some form of flow augmentation procedure will be necessary if the wound is to heal and the limb salvaged. If regional ischemia is modest, or if the problem is chiefly microvascular in nature, hyperbaric oxygen becomes a therapeutic option.
Of the several methods that evaluate vascular competence, transcutaneous pO2 monitoring appears best suited to guide hyperbaric decision making. In contrast with pressure and volume assessments, transcutaneous pO2 monitoring precisely measures the “end point” of the oxygen delivery system, i.e., oxygen tensions present within the skin or subjacent tissues.
Threshold oxygen tensions have been established for healthy [30,31] and critically ischemic [32] tissues. Transcutaneous pO2 directly reflects the indication for hyperbaric oxygen therapy and any subsequent therapeutic effect. Furthermore it provides information regarding the relative contributions of macro and microvascular flow disruption. First introduced as a non-invasive assessment of arterial oxygen tension in neonates [33], transcutaneous oxygen mapping has been employed in vascular [34], orthopedic [35,36], and plastic surgery [37] settings.
Most recently, the importance of transcutaneous pO2 measurement in the management of distal lower extremity disease has been prospectively demonstrated [38,39,40]. Its value in predicting the risk of amputation in the diabetic population is very evident [41].
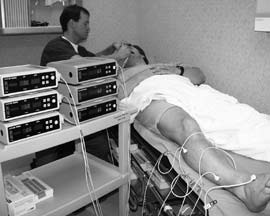 |
FIG 2: tcpO2 hyperbaric decision making. |
For the hyperbaricist, tcpO2 is critical to the successful evaluation and management of problem wound referrals. As a number of local and systemic factors are known to adversely influence wound healing, it is important to identify underlying hypoxia if hyperbaric oxygen therapy is to be effectively applied. As several etiologies may exist in the same patient, one must work up each patient comprehensively and manage accordingly.
Transcutaneous pO2 monitoring is employed algorithmically throughout the evaluation and case management periods.
TRANSCUTANEOUS OXYGEN MANAGEMENT ALGORITHM
Hyperbaric wound healing referrals undergo a comprehensive work-up, including a detailed medical history, physical examination, and selected diagnostic testing. Baseline transcutaneous oxygen screening is followed up in an algorithmic manner in those patients whose risk-benefit ratio is in favor of a trial of hyperbaric oxygen therapy. The algorithm addresses four essential questions:
I Is wound healing complicated by hypoxia?
II When present, is hypoxia reversible?
III Is the patient responding to hyperbaric oxygen therapy?
IV Has the patient reached a therapeutic end point?
I: Is wound healing complicated by hypoxia?
- Normal lower extremity tcpO2 values exceed 50 mmHg when recorded at one atmosphere absolute (760 mmHg) [39,40].
- Values ranging from 35-40 mmHg and higher are considered sufficient to support oxygen-dependent wound healing [28,39,41,42].
- Values below this range represent a risk of healing compromise, the degree of which increases as values decrease [42].
II: When present, is hypoxia reversible?
For hyperbaric oxygen (a systemic method of dose delivery) to be effective, a certain degree of regional perfusion must be present.
- Breathing 100 % oxygen at normobaric pressure following the recording of a steady-state ambient tcpO2 value evaluates the state of regional arterial flow.
- Oxygen challenge values in excess of 300 mmHg represent essentially uncompromised regional perfusion.
- Screening values in excess of approximately 100 mmHg are suggestive of adequate regional perfusion for limb viability and reflect a suitable hyperbaric candidate.
- Screening values that fail to reach 100 mmHg are consistent with significant ischemia and warrant further vascular work-up. The decision to incorporate hyperbaric oxygen therapy into the treatment plan is made on a case-by-case basis and following decisions regarding any flow augmentation options.
III: Is the patient responding to hyperbaric oxygen therapy?
The above patient selection process does not predict outcome. It identifies those patients who have the physiologic capacity to deliver high pressures of oxygen to the wound margin. There has been an effort to incorporate tcpO2 as an outcome predictor [43]. This is an unresolved issue, however [44].
It is probably asking too much of this technology, for the diabetic patient in particular and given the complexity of such lesions. Consequently, early evidence of therapeutic response is sought. Improvement in ambient (21 % O2) tcpO2 over time has consistently been the best indicator of therapeutic response [44]. The absence of such a response might alert the clinician to a potential non-responder.
This should permit evaluation for other possible impediments to wound repair, thereby avoiding an otherwise unsuccessful and expensive course of therapy.
Hyperoxic-induced angiogenic responses have been monitored transcutaneously in tissues rendered ischemic secondary to therapeutic radiation [16]. A distinct “rapid rise phase” in tcpO2 occurs following 8-10 treatments. This rise tends to plateau at 20-22 treatments. As a point of re-evaluation we have selected 14 treatments, essentially midway through this period of rapid change in tcpO2. If neovascularization is taking place, there should be improved baseline (21 % O2) periwound values at this point.
- If values are increasing, the patient is considered a responder, and hyperbaric treatments are continued to Step IV.
- If there has been no change or if deterioration is evident, the patient undergoes further work-up for etiologies other than hypoxia. Hyperbaric oxygen therapy may be held at this point.
The goal of Step III is to avoid lengthy and ultimately unsuccessful courses of hyperbaric oxygen therapy.
IV: Has the patient reached a therapeutic end point?
In this era of healthcare reform with efforts to contain costs, greater scrutiny is being directed at the healthcare delivery system in general, and those modalities not entirely entrenched within mainstream medical practice in particular. It is important, therefore, that the decision to utilize hyperbaric oxygen therapy be mediated, in part, by its financial impact.
In carefully selected patients, managed along algorithmic and evidence-based lines, hyperbaric oxygen therapy provides excellent and enduring clinical outcomes while reducing the total healthcare cost. When used in a non-discriminating manner, it is expensive and of questionable clinical value.
In terms of the wound healing referral, transcutaneous oxygen monitoring holds great promise as a cost containment tool. It is already noted that patient selection today is much more discriminating. Well-oxygenated chronic wounds are directed to management strategies other than hyperbaric oxygenation. Hypoxic wounds that are the consequence of high-grade regional ischemia are likewise referred from the hyperbaricist to the vascular service.
In those patients entered into a hyperbaric treatment protocol, non-responders are identified early rather than following many weeks or even months of treatment.
The final step is to identify when the prescribed course of hyperbaric oxygen therapy has produced sufficient angiogenesis to support spontaneous healing. It is neither necessary nor cost-effective to treat such wounds to complete resolution.
Once the environment around the wound has been “normalized” and the patient converted to a locally host-competent state, hyperbaric oxygen can be stopped. Periwound transcutaneous oxygen values that reach or exceed 40 mmHg suggest adequate neovascularization has been formed. Typically, clinical evidence of healing responses will be apparent at this time, however, the wound may not be fully healed.
At this point hyperbaric oxygen therapy can be stopped. Standard wound care measures remain in force, and the patient is followed for continued healing responses. If the wound plateaus or regresses, hyperbaric oxygen therapy is reinstituted.
In the setting for which this protocol is designed i.e., the chronic and refractory skin ulceration, withholding hyperbaric therapy for one or two weeks is unlikely to represent a limb-threatening event.
COST-BENEFIT IMPLICATIONS
The healthcare profession remains under increasing pressure to maximize clinical outcomes while containing expenditures. As with other specialized medical technologies, HBO therapy is firmly under the “outcomes microscope”. To be deemed a clinical and cost-effective intervention, HBO therapy must be practiced in a selective and judicious manner.Treatments are expensive. In the United States, the average cost of a single treatment (combining hospital and physician fees) is of the order of USD 650.00. In the case of severe carbon monoxide poisoning involving just one or two treatments, this may not seem an unreasonable cost. On the other end of the spectrum is the problem wound referral. If poorly managed, these cases may involve very lengthy courses of treatment that are difficult to justify on both clinical and financial grounds.
Transcutaneous pO2 guides the hyperbaricist by providing an “evidence-based” element of decision making. Where chronic and refractory wounds are found to involve reversible hypoxia, a trial of HBO therapy would appear indicated. Nonhypoxic wounds and hypoxic wounds involving high-grade regional ischemia are referred on to those who can best address macrovascular flow. Patients who begin HBO therapy are followed closely for early evidence of healing responses, then “normalization” of local hypoxia.
The application of the above management algorithm has had a significant impact at our institution. A marked reduction in the total number of hyperbaric treatments provided for wound healing referrals has resulted, while clinical outcomes have continued to improve.
This approach has not been lost on the health insurance industry. It is becoming increasingly common for insurers to question the diagnosis of hypoxia-mediated healing compromise and the need for hyperbaric oxygen therapy. Transcutaneous oxygen studies play a key role in substantiating the diagnosis and subsequent medical decision making.
In a very recent development, Medicare has published a list of “indications for effective treatment outcomes for HBO” therapy [42]. One such indication is “resolved hypoxia”, which is further defined as oxygen levels greater than 40 mmHg. Medicare is the US Government’s program that provides health benefits to the elderly. Medicare beneficiaries make up the majority of patients treated hyperbarically, so this definition which goes on to state that “the body can now resume most functions of wound healing and antimicrobial defenses without need of HBO” has an enormous implication.
It demands the application of the management algorithm described herein.
References+ View more
- Tibbles PM, Edelsberg JS. N Engl J Med 1996; 334, 25: 1642-48.
- Boykin JV. Wounds 1996; 8, 6: 183-98.
- Hammarlund C, Sundberg T. Plastic and Reconstructive Surgery 1994; 93, 4: 829-34.
- Matos LA, Numez AA. In: E. P. Kindwall, ed. Hyperbaric Medicine Practice. Flagstaff, Arizona: Best Publishing Co., 1994.
- Pecoraro RE, Ahroni JH, Boyko EJ, et al. Diabetes 1991; 40: 1305-13.
- Lavan FB, Hunt TK. Clinics in Plastic Surgery 1990; 17, 3: 463-72.
- Mader J, Brown GL, Gucklan JC. The Journal of Infectious Diseases 1980; 142, 6: 915-22.
- Siddiqui A, Davidson JD, Mustoe TA. Plastic and Reconstructive Surgery 1997; 99: 148-55.
- Tompach PC, Lew D, Stoll JL. Int. J. Oral Maxillofac. Surg. 1997; 26: 82-86.
- Hehenberger K, Brismar K, Lind F, et al. Wound Repair and Regeneration 1997; 5: 147-50.
- Hyperbaric Oxygen Committee Report. Kensington, Maryland, Undersea and Hyperbaric Medical Society, 1996.
- Leslie CA, Sapico FL, Ginunas VJ, et al. Diabetes Care 1988; 11, 2: 111-15.
- Marx RE, Johnson RP. In: Problem wounds: The role of oxygen. Davis JC and Hunt TK, eds. New York: Elsevier, 1988.
- Moon RE. Problems in Respiratory Care 1991; 4, 2: 232-52.
- Fife CE, Camporesi EM. Problems in Respiratory Care 1991; 4, 2: 142-49.
- Marx RE, Ehler WJ, Tayapongsak P, et al. American J Surg 1990; 160: 519-24.
- Nylander G, Lewis D, Nordstrom H, et al. Plastic and Reconstructive Surgery 1985;76, 4: 596-601.
- Wells CH, Goodpasture JE, Horrigan D, et al. Proceedings of the 6th International Congress on Hyperbaric Medicine 1997: 118-24.
- Zamboni WA, Roth AC, Russell RC, et al. Plastic and Reconstructive Surgery 1993; 91, 6: 1110-23.
- Faglia E, Favales F, Aldeghi A, et al. Diabetes Care 1996; 19, 12: 1338-43.
- Bouachour MD, Cronier P, Gouello JP, et al. The Journal of Trauma: Injury, Infection, and Critical Care 1996; 41, 2: 333-39.
- Boykin JV, Crossland MC, Cole LM. Journal of Healthcare Resource Management 1997; May: 22-26.
- Cianci P, Hunt TK. Wound Rep. Reg. 1997; 5: 141-46.
- Thom SR, Taber R, Mendiguren I, et al. Annals of Emergency Medicine 1995; 25, 4: 474-80.
- Helps SC, Gorman DF. Stroke 1991; 22: 351-354.
- Bakker DJ, Van der Kleu AJ. Handbook on hyperbaric medicine, 1996: 362-85.
- Hollabaugh RS, Dmochowski RR, Hickerson WL, et al. Plastic and Reconstructive Surgery 1998; 101: 94-100.
- Cianci PE, Sato R. Burns 1994; 20, 1: 5-14.
- Smith D, et al. J. Gen. Intern Med. 1987; 2: 232-38.
- Dowd GS, Linge K, Bentley G. The Journal of Bone and Joint Surgery 1983; 65-B, 1: 79-83.
- Orenstein A, Mazkereth R, Tsur H. Journal of Plastic Surgery 1988; 20, 5: 419-25.
- Padberg FT, Back TL, Thompson PN, et al. J Surg Res 1996; 60, 2: 365-69.
- Huch R, Huch A. Crit Care Med 1981; 9, 10: 694-97.
- Rooke TW. Int Ang 1992; 11, 1: 36-40.
- Wyss CR, Harrington RM, Burgess EM, et al. The Journal of Bone and Joint Surgery 1988; 70-A, 2: 203-07.
- Harward TR, Volny J, Golbranson F, et al. J Vasc Surg 1985; 2: 220-27.
- Achauer BM, Black KS, Litke DK. Plastic and Reconstructive Surgery 1980; 65, 6: 738-45.
- Ballard JL, Eke CC, Bunt TJ, et al. J. Vasc. Surgery 1995; 22: 485-92.
- Bunt TJ, Holloway GA. Ann Vasc Surg 1996; 10, 3: 224-27.
- Hanna GP, Fujise K, Kjellgren O, et al. Journal of American College of Cardiology 1997; 30: 664-69.
- Reiber G, Pecoraro R, Koepsell T. Ann of Intern Med 1992; 117, 2: 97-105.
- Medicare Review Policy No. 98A-0016-L, 1998. Medicare Part A Medical Policy.
- Wattel F, Mathieu D, Coget JM. Proceedings, 2nd European Conference on Hyperbaric Medicine, Basel, 1990: 221-23.
- Dooley J, Schirmer J, Slade B, et al. Undersea Hyperbaric Med, 1996; 23, 3: 167-74.
References
- Tibbles PM, Edelsberg JS. N Engl J Med 1996; 334, 25: 1642-48.
- Boykin JV. Wounds 1996; 8, 6: 183-98.
- Hammarlund C, Sundberg T. Plastic and Reconstructive Surgery 1994; 93, 4: 829-34.
- Matos LA, Numez AA. In: E. P. Kindwall, ed. Hyperbaric Medicine Practice. Flagstaff, Arizona: Best Publishing Co., 1994.
- Pecoraro RE, Ahroni JH, Boyko EJ, et al. Diabetes 1991; 40: 1305-13.
- Lavan FB, Hunt TK. Clinics in Plastic Surgery 1990; 17, 3: 463-72.
- Mader J, Brown GL, Gucklan JC. The Journal of Infectious Diseases 1980; 142, 6: 915-22.
- Siddiqui A, Davidson JD, Mustoe TA. Plastic and Reconstructive Surgery 1997; 99: 148-55.
- Tompach PC, Lew D, Stoll JL. Int. J. Oral Maxillofac. Surg. 1997; 26: 82-86.
- Hehenberger K, Brismar K, Lind F, et al. Wound Repair and Regeneration 1997; 5: 147-50.
- Hyperbaric Oxygen Committee Report. Kensington, Maryland, Undersea and Hyperbaric Medical Society, 1996.
- Leslie CA, Sapico FL, Ginunas VJ, et al. Diabetes Care 1988; 11, 2: 111-15.
- Marx RE, Johnson RP. In: Problem wounds: The role of oxygen. Davis JC and Hunt TK, eds. New York: Elsevier, 1988.
- Moon RE. Problems in Respiratory Care 1991; 4, 2: 232-52.
- Fife CE, Camporesi EM. Problems in Respiratory Care 1991; 4, 2: 142-49.
- Marx RE, Ehler WJ, Tayapongsak P, et al. American J Surg 1990; 160: 519-24.
- Nylander G, Lewis D, Nordstrom H, et al. Plastic and Reconstructive Surgery 1985;76, 4: 596-601.
- Wells CH, Goodpasture JE, Horrigan D, et al. Proceedings of the 6th International Congress on Hyperbaric Medicine 1997: 118-24.
- Zamboni WA, Roth AC, Russell RC, et al. Plastic and Reconstructive Surgery 1993; 91, 6: 1110-23.
- Faglia E, Favales F, Aldeghi A, et al. Diabetes Care 1996; 19, 12: 1338-43.
- Bouachour MD, Cronier P, Gouello JP, et al. The Journal of Trauma: Injury, Infection, and Critical Care 1996; 41, 2: 333-39.
- Boykin JV, Crossland MC, Cole LM. Journal of Healthcare Resource Management 1997; May: 22-26.
- Cianci P, Hunt TK. Wound Rep. Reg. 1997; 5: 141-46.
- Thom SR, Taber R, Mendiguren I, et al. Annals of Emergency Medicine 1995; 25, 4: 474-80.
- Helps SC, Gorman DF. Stroke 1991; 22: 351-354.
- Bakker DJ, Van der Kleu AJ. Handbook on hyperbaric medicine, 1996: 362-85.
- Hollabaugh RS, Dmochowski RR, Hickerson WL, et al. Plastic and Reconstructive Surgery 1998; 101: 94-100.
- Cianci PE, Sato R. Burns 1994; 20, 1: 5-14.
- Smith D, et al. J. Gen. Intern Med. 1987; 2: 232-38.
- Dowd GS, Linge K, Bentley G. The Journal of Bone and Joint Surgery 1983; 65-B, 1: 79-83.
- Orenstein A, Mazkereth R, Tsur H. Journal of Plastic Surgery 1988; 20, 5: 419-25.
- Padberg FT, Back TL, Thompson PN, et al. J Surg Res 1996; 60, 2: 365-69.
- Huch R, Huch A. Crit Care Med 1981; 9, 10: 694-97.
- Rooke TW. Int Ang 1992; 11, 1: 36-40.
- Wyss CR, Harrington RM, Burgess EM, et al. The Journal of Bone and Joint Surgery 1988; 70-A, 2: 203-07.
- Harward TR, Volny J, Golbranson F, et al. J Vasc Surg 1985; 2: 220-27.
- Achauer BM, Black KS, Litke DK. Plastic and Reconstructive Surgery 1980; 65, 6: 738-45.
- Ballard JL, Eke CC, Bunt TJ, et al. J. Vasc. Surgery 1995; 22: 485-92.
- Bunt TJ, Holloway GA. Ann Vasc Surg 1996; 10, 3: 224-27.
- Hanna GP, Fujise K, Kjellgren O, et al. Journal of American College of Cardiology 1997; 30: 664-69.
- Reiber G, Pecoraro R, Koepsell T. Ann of Intern Med 1992; 117, 2: 97-105.
- Medicare Review Policy No. 98A-0016-L, 1998. Medicare Part A Medical Policy.
- Wattel F, Mathieu D, Coget JM. Proceedings, 2nd European Conference on Hyperbaric Medicine, Basel, 1990: 221-23.
- Dooley J, Schirmer J, Slade B, et al. Undersea Hyperbaric Med, 1996; 23, 3: 167-74.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Transcutaneous oxygen monitoring of hyperbaric problem wound referrals
Presented by Dick Clarke, CHT. President, National Baromedical Services, Inc. President, National Board of Diving and Hyperbaric Medical Technology. Watch the webinarRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar