Printed from acutecaretesting.org
April 2009
Biomarkers in sepsis: the present and the future
INTRODUCTION
Bacterial infections and sepsis are major causes of morbidity and mortality in hospitalized patients [1-3]. Accurate and timely diagnosis of infection remains challenging.
Clinical and laboratory signs of systemic inflammation, including changes in body temperature, tachycardia, respiratory rate and leucocytosis, are sensitive.
However, their use is limited by poor specificity for the diagnosis of sepsis because critically ill patients often present with the systemic inflammatory response syndrome (SIRS) but no infection [1,4-6].
These issues have fueled the search for a reliable marker. Many potential biomarkers have been investigated, but only C-reactive protein (CRP) and procalcitonin (PCT) are currently used on a routine basis [7-10].
These two biomarkers are definitely the two most investigated, and they will probably remain important sepsis biomarkers for many years. However, the search for a single "magic bullet" marker might ultimately be fruitless, but a combination of markers could improve diagnosis, prognosis and treatment efficacy.
In the field of cardiology biomarkers are pivotal to answering similar questions in relation to acute myocardial infarction (e.g. troponin), and the use of biomarkers in risk algorithms has dramatically reduced mortality in this disease [11]. Hopefully, sepsis biomarkers will play the same important role in improving future sepsis outcome.
In this paper I review recent advances with the use of biomarkers in diagnosis and management of sepsis patients, with emphasis on biomarkers that may be introduced in the clinic during the next years. At the same time I discuss possible future developments in sepsis biomarkers.
THE PERFECT SEPSIS BIOMARKER
What do clinicians and clinical trials demand of a "perfect" sepsis biomarker?
There are several important characteristics: First of all it should be highly sensitive and specific for sepsis to allow the differentiation between infectious and non-infectious causes of inflammation, organ dysfunction and shock; secondly, it should be present at the onset or even before the appearance of the clinical signs of sepsis to have prognostic value; thirdly, it should be easy and safe to measure with low cost for the patients and for the hospital; finally, it should be biologically plausible.
STATISTICAL ASSESSMENT OF SEPSIS-BIOMARKER PERFORMANCE
It is practically impossible to avoid the receiver-operator characteristic (ROC) curve when writing an article about sepsis biomarkers. The ROC curve is a widely used tool for comparing diagnostic tests.
The curve is constructed by plotting the diagnostic sensitivity and specificity values for every individual cut-off on a graph with 1-specificity on the x-axis and sensitivity on the y-axis (Figure 1).
The shape of an ROC curve and the area under the curve (AUC) helps estimate the discriminative power of a marker. The closer the curve is located to the upper left-hand corner and the larger the AUC, the better the marker is at discriminating between septic and non-septic patients.
A perfect biomarker has an AUC of 1, whereas a non-discriminating marker has an area of 0.5.
FIGURE 1: An example of receiver operator characteristic (ROC) curves
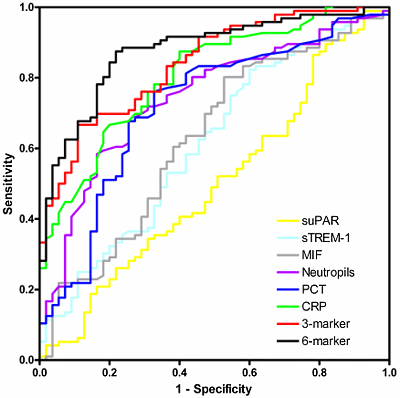
Displayed are ROC curves comparing soluble urokinase-type plasminogen activator receptor (suPAR), soluble triggering receptor expressed on myeloid cells (sTREM)-1, macrophage migration inhibitory factor (MIF), neutrophil count, procalcitonin (PCT), C-reactive protein (CRP), and the combined three-marker and six-marker tests for detection of bacterial versus non-bacterial causes of systemic inflammation.
The six-marker is the best-performing marker (ROC-AUC 0.88) and suPAR is in this comparison as good as a toss of a coin with (ROC-AUC 0.50).
The figure was first published in: Kofoed K, Andersen O, Kronborg G, el al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care 2007; 11: R38.
SEPSIS BIOMARKERS IN USE
The list of proposed sepsis biomarkers is indeed long. Nevertheless, only a couple have gained widespread use.
C-reactive protein
CRP is measured in thousands of clinics around the world. CRP is an acute-phase protein synthesized in hepatocytes and alveolar macrophages [12] in response to a variety of cytokines, in particular IL-6. CRP has both pro- and anti-inflammatory effects.
Serum CRP is attractive as a biomarker because plasma concentrations increase rapidly in response to inflammation and half-life is short (19 hours), although not as short as that of PCT [13,14]. Finally, most CRP assays are inexpensive.
Procalcitonin
PCT is also measured in several hospitals, especially in ICUs. PCT is a propeptide of calcitonin that is ubiquitously expressed as part of the inflammatory response to a range of insults [15,16]. During the last years several new platforms for the measurement of PCT have been introduced, and as for CRP bedside models are now available.
Studies investigating the use of PCT and CRP have found the diagnostic performance of CRP and PCT to be rather similar [17-20]. With regard to diagnosing bacteremia in particular, PCT have shown excellent diagnostic ability; this is in accordance with the suggested notion that PCT is superior to CRP in diagnosing systemic infection [17,19-22].
What makes PCT particularly interesting is that several well-designed studies have proven that algorithms based on PCT concentrations as the main guide can shorten the length of antibiotic treatment and decrease the use of antibiotics [23-25]. The data analysis from a study using a PCT-guided algorithm to improve survival in ICU patients is awaited with great expectations.
FUTURE SEPSIS BIOMARKERS
Increasing insight into the function and signal pathways of the innate immune system has highlighted a limited number of biologically plausible sepsis biomarkers measurable in human plasma.
Triggering receptor expressed on myeloid cells-1
TREM are a group of cell-surface receptors that belong to the immunoglobulin superfamily [26]. TREM-1 is expressed mainly on macrophages and neutrophils, and has been identified as an amplifier of the immune response that strongly enhances leukocyte activation in the presence of microbial products [26,27].
Levels of TREM-1 at the cell surface are up-regulated in the presence of bacteria or fungi [27,28]; however, non-microbial stimuli (e.g. urate crystals) have also been shown to enhance the expression of TREM-1 [29,30]. Despite several investigations, the nature of TREM-1 ligand(s) remains elusive.
In addition to the membrane-bound form, a soluble TREM-1 variant (sTREM-1) has been detected in several body fluids [31-33]. Recently published findings strongly suggest that proteolysis of the membrane-anchored TREM-1 is the only source of sTREM-1 [34].
Initially, sTREM-1 was only found in fluids from patients with microbial infections [29,31,32,35], but recent studies have found elevated sTREM-1 plasma levels in patients with non-infectious conditions, such as inflammatory bowel disease and chronic obstructive pulmonary disease, and in patients undergoing cardiac surgery [20,36-38].
The latter results suggest that sTREM-1 can be released by a broad spectrum of inflammatory stimuli.
The first promising result of the use of sTREM-1 in plasma to diagnose sepsis in ICU patients [31] indicated that sTREM-1 might be that perfect diagnostic sepsis biomarker that everybody had been looking for.
Several studies on the use of sTREM-1 to diagnose a variety of infections have followed. The diseases for which the diagnostic accuracy of sTREM-1 has been most extensively investigated is pneumonia and sepsis.
With regard to setting, the majority of studies have been performed in ICUs and only a few of the studies included more than 150 patients. The heterogeneity in study design and setting, and the limited size of the studies published make it impossible to draw firm conclusions on the diagnostic accuracy of sTREM-1.
Results are ranging from an accuracy of almost 100 % (an ROC-AUC of 0.97) for the diagnosis of sepsis in ICU patients with SIRS [31] to an accuracy as good as a toss of a coin in a study published this month [40]. Measurement of sTREM-1 in bronchoalveolar lavage (BAL) fluid might provide more reliable results [32].
However, the measurement of sTREM-1 in BAL fluid is not feasible in the routine care of patients suspected of infection, but might be practicable in an ICU setting. A recently published meta-analysis of the diagnostic value of sTREM-1 concluded that sTREM-1 represents a reliable biological marker of bacterial infection [41].
Cytokines
The initial host-microbial pathogen interaction is followed by an activation of other parts of the innate immune response to coordinate a defensive response involving both humoral and cellular components.
Mononuclear cells play a key role, releasing the long-known pro-inflammatory cytokines interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and other cytokines, including IL-12, IL-15 and macrophage migration inhibitory factor (MIF) [42,43].
This pro-inflammatory cascade is mediated by direct cell-to-cell interactions and by soluble factors derived from serum proteins and cells such as complement system proteins and cytokines. It is important to acknowledge that the increased expression of pro-inflammatory molecules is only part of this evolving immunological response.
Anti-inflammatory modulators, such as IL-1 receptor antagonist, IL-10 and soluble TNF-α receptors I and II play an important role [44]. Anti- and pro-inflammatory molecules coexist in the circulation in patients with established sepsis and presumably within the tissues [44].
Thus, severe sepsis may be more accurately described as a dysregulation of the innate immune system, rather than just the over-expression of either pro- or anti-inflammatory substances. This network of combined pro- and anti-inflammatory mediator interaction may in part explain the failures of the past decade’s major anti-inflammatory drug trials in the treatment of septic [10,45,46].
Having a pivotal role in the sepsis response, cytokines could be important sepsis biomarkers. However, the single measurement of most cytokines has proven to be insufficient for distinguishing between infected and non-infected patients.
Some evidence suggests that IL-6 performs acceptably well, especially in neonates [47-50]. An ROC-AUC of 0.75 has been reported for distinguishing SIRS from sepsis [50].
Based on our present knowledge, measurements of single cytokines will not have great impact on the future diagnosis of sepsis. However, it is plausible that real-time monitoring of a panel of cytokines and receptors will be important to determine the level of dysregulation of the innate immune system and to guide future sepsis treatments.
An essential requirement of any real-time monitoring system is patient proximity, with samples being analyzed in an ICU or ED setting rather than in a centralized hospital laboratory.
BIOMARKER COMBINATIONS
Sepsis can be caused by numerous pathogens and the primary site of infection can be any major organ system; thus no single marker may be able to have the high accuracy needed for fast and accurate guidance of treatment of sepsis patients.
Therefore, the search for a single "magic bullet" marker might ultimately be fruitless, but a combination of markers could improve diagnosis, prognosis and treatment efficacy, and thereby survival [51]. Instead of a single marker, a combination of markers may be the right approach to crack the "sepsis code".
In 2007 my colleagues and I published results of constructed composite diagnostic markers in a cohort of patients admitted from the community with SIRS and suspected infection [20].
We evaluated soluble urokinase plasminogen activator receptor, sTREM-1, and MIF using multiplex assays, along with standard measurement of CRP, PCT and neutrophil count. A combination of the three best-performing markers (CRP, PCT and neutrophil count) and all six markers were found to be more accurate in detecting inflammatory response caused by bacterial infection than individual markers alone with an ROC-AUC of 0.88 (Figure 1).
Two months ago Shapiro et al published results from a multicenter study of ED patients with suspected sepsis [52]. Nine biomarkers were assayed and multivariable logistic regression was used to identify an optimum combination of biomarkers to create a panel. Among the nine biomarkers tested, the optimum three-marker panel was neutrophil gelatinase-associated lipocalin, protein C and interleukin-1 receptor antagonist.
The ROC-AUC for the accuracy of the sepsis score derived from these three biomarkers was 0.80 for severe sepsis, 0.77 for septic shock and 0.79 for death. In the future, multimarker panels will probably add to the diagnostic accuracy and risk assessment in sepsis. However, validation studies are needed to show that the marker combinations can be used in other settings than the ones they are tested in.
CONCLUSIONS
Accurate and timely diagnosis of infection and monitoring of treatment effects are very important for patient outcomes. To aid this process there is a need for reliable sepsis biomarkers and a real-time monitoring system.
Increasing insight into the function and signal pathways of the innate immune system has highlighted a limited number of biologically plausible sepsis biomarkers measurable in human plasma. However, few have been tested in clinical settings, and only PCT has been tested in randomized trials.
Current evidence suggests that CRP will remain an important marker of inflammation and infection, and that PCT will enhance the clinicians’ ability to diagnose infection in critically ill patients and probably guide therapy. Thus I foresee that PCT will be measured in more patients in the future.
A sepsis biomarker that has attracted a lot of attention during the last years is sTREM-1. On the one hand there are studies showing that sTREM-1 is the ideal sepsis biomarker, and on the other hand there are studies showing that sTREM-1 is as accurate as a toss of a coin.
The accuracy is, as is the case with other biomarkers, highly dependent on the setting and the gold standard against which the marker is tested, but sTREM-1 is probably not the perfect sepsis biomarker that everyone is looking for.
Given the complexity and variability of sepsis it is understandable that no single biomarker possesses all of the "perfect biomarker" qualities. Combining information from several sepsis markers is simple and may facilitate diagnosis and risk assessment in septic patients.
References+ View more
- Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348: 1546-54.
- Annane D, Aegerter P, Jars-Guincestre MC, et al. Current epidemiology of septic shock: the CUBRèa Network. Am J Respir Crit Care Med 2003; 168: 165-72.
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101: 1644-55.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345: 1368-77.
- Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med 2005; 33: 2194-201.
- Kumar A, Roberts D, Wood K, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in septic shock. Crit Care Med 2006; 34: 1589-96.
- The Biomarker Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89-95.
- Wagner JA, Williams SA, Webster CJ. Biomarkers and surrogate end points for fit-for-purpose development and regulatory evaluation of new drugs. Clin Pharmacol Ther 2007; 81: 104-07.
- Lee JW, Devanarayan V, Barrett YC, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res 2006; 23: 312-28.
- Marshall JC, Vincent JL, Fink MP, et al. Measures, markers, and mediators: toward a staging system for clinical sepsis: a report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25-26, 2000. Crit Care Med 2003; 31: 1560-67.
- Anwaruddin S, Askari AT, Topol EJ. Redefining risk in acute coronary syndromes using molecular medicine. J Am Coll Cardiol 2007; 49: 279-89.
- Dong Q, Wright JR. Expression of C-reactive protein by alveolar macrophages. J Immunol 1996; 156: 4815-20.
- Claeys R, Vinken S, Spapen H, et al. Plasma procalcitonin and C-reactive protein in acute septic shock: clinical and biological correlates. Crit Care Med 2002; 30: 757-62.
- Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med 1998; 24: 888-89.
- Becker KL, Nylén ES, White JC, et al. Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 2004; 89: 1512-25.
- Wanner GA, Keel M, Steckholzer U, et al. Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit Care Med 2000; 28: 950-57.
- Chan YL, Tseng CP, Tsay PK, et al. Procalcitonin as a marker of bacterial infection in the emergency department: an observational study. Crit Care 2004; 8: R12-R20.
- Gaini S, Koldkjaer OG, Pedersen C, et al. Procalcitonin, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in community-acquired infections and sepsis: a prospective study. Crit Care 2006; 10: R53.
- Hausfater P, Garric S, Ayed SB, et al. Usefulness of procalcitonin as a marker of systemic infection in emergency department patients: a prospective study. Clin Infect Dis 2002; 34: 895-901.
- Kofoed K, Andersen O, Kronborg G, el al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care 2007; 11: R38.
- Chirouze C, Schuhmacher H, Rabaud C, et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis 2002; 35: 156-61.
- Ugarte H, Silva E, Mercan D, et al. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med 1999; 27: 498-504.
- Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 2004; 363: 600-07.
- Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008 Oct 13; 168: 2000-07.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007; 131: 9-19.
- Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol 2006; 7: 1266-73.
- Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 2000; 164: 4991-95.
- Bouchon A, Facchetti F, Weigand MA, et al. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001; 410: 1103-07.
- Schenk M, Bouchon A, Seibold F, et al. TREM-1--expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest 2007; 117: 3097-3106.
- Murakami Y, Akahoshi T, Hayashi I, et al. Induction of triggering receptor expressed on myeloid cells 1 in murine resident peritoneal macrophages by monosodium urate monohydrate crystals. Arthritis Rheum 2006; 54: 455-62.
- Gibot S, Kolopp-Sarda MN, Bene MC, et al. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med 2004; 141: 9-15.
- Gibot S, Cravoisy A, Levy B, et al. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med 2004; 350: 451-58.
- Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis 2003; 187 Suppl 2: S397-S401.
- Gomez-Pina V, Soares-Schanoski A, Rodriguez-Rojas A, et al. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol 2007; 179: 4065-73.
- Gibot S, Cravoisy A, Kolopp-Sarda MN, et al. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med 2005; 33: 792-96.
- Phua J, Koay ES, Zhang DH, et al. Soluble triggering receptor expressed on myeloid cells-1 in acute respiratory infections. Eur Respir J 2006; 28: 695-702.
- Tzivras M, Koussoulas V, Giamarellos-Bourboulis EJ, et al. Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease. World J Gastroenterol 2006; 12: 3416-19.
- Adib-Conquy M, Monchi M, Goulenok C, et al. Increased plasma levels of soluble triggering receptor expressed on myeloid cells 1 and procalcitonin after cardiac surgery and cardiac arrest without infection. Shock 2007; 28: 406-10.
- Giamarellos-Bourboulis EJ, Zakynthinos S, Baziaka F, et al. Soluble triggering receptor expressed on myeloid cells 1 as an anti-inflammatory mediator in sepsis. Intensive Care Med 2006; 32: 237-43.
- Bopp C, Hofer S, Bouchon A, el at. Soluble TREM-1 is not suitable for distinguishing between systemic inflammatory response syndrome and sepsis survivors and nonsurvivors in the early stage of acute inflammation. Eur J Anaesthesiol 2009 Mar 20 [Epub ahead of print].
- Jiyong J, Tiancha H, Wei C, et al. Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in bacterial infection: a meta-analysis. Intensive Care Med 2009; 35: 587-95.
- Martins PS, Brunialti MK, da Luz FM, et al. Bacterial recognition and induced cell activation in sepsis. Endocr Metab Immune Disord Drug Targets 2006; 6: 183-91.
- Roger T, David J, Glauser MP, et al. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 2001; 414: 920-24.
- Goldie AS, Fearon KC, Ross JA, et al. Natural cytokine antagonists and endogenous antiendotoxin core antibodies in sepsis syndrome. The Sepsis Intervention Group. JAMA 1995; 274: 172-77.
- Opal SM, Cross AS. Clinical trials for severe sepsis. Past failures, and future hopes. Infect Dis Clin North Am 1999; 13: 285-97, vii.
- Abraham E. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med 1999; 25: 556-66.
- Oda S, Hirasawa H, Shiga H, et al. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine 2005; 29: 169-75.
- Chiesa C, Pellegrini G, Panero A, et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem 2003; 49: 60-68.
- Ventetuolo CE, Levy MM. Biomarkers: diagnosis and risk assessment in sepsis. Clin Chest Med 2008; 29: 591-603.
- Harbarth S, Holeckova K, Froidevaux C, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med 2001; 164: 396-402.
- Carrigan SD, Scott G, Tabrizian M. Toward resolving the challenges of sepsis diagnosis. Clin Chem 2004; 50: 1301-14.
- Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med 2009; 37: 96-104.
References
- Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348: 1546-54.
- Annane D, Aegerter P, Jars-Guincestre MC, et al. Current epidemiology of septic shock: the CUBRèa Network. Am J Respir Crit Care Med 2003; 168: 165-72.
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101: 1644-55.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345: 1368-77.
- Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med 2005; 33: 2194-201.
- Kumar A, Roberts D, Wood K, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in septic shock. Crit Care Med 2006; 34: 1589-96.
- The Biomarker Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89-95.
- Wagner JA, Williams SA, Webster CJ. Biomarkers and surrogate end points for fit-for-purpose development and regulatory evaluation of new drugs. Clin Pharmacol Ther 2007; 81: 104-07.
- Lee JW, Devanarayan V, Barrett YC, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res 2006; 23: 312-28.
- Marshall JC, Vincent JL, Fink MP, et al. Measures, markers, and mediators: toward a staging system for clinical sepsis: a report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25-26, 2000. Crit Care Med 2003; 31: 1560-67.
- Anwaruddin S, Askari AT, Topol EJ. Redefining risk in acute coronary syndromes using molecular medicine. J Am Coll Cardiol 2007; 49: 279-89.
- Dong Q, Wright JR. Expression of C-reactive protein by alveolar macrophages. J Immunol 1996; 156: 4815-20.
- Claeys R, Vinken S, Spapen H, et al. Plasma procalcitonin and C-reactive protein in acute septic shock: clinical and biological correlates. Crit Care Med 2002; 30: 757-62.
- Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med 1998; 24: 888-89.
- Becker KL, Nylén ES, White JC, et al. Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 2004; 89: 1512-25.
- Wanner GA, Keel M, Steckholzer U, et al. Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit Care Med 2000; 28: 950-57.
- Chan YL, Tseng CP, Tsay PK, et al. Procalcitonin as a marker of bacterial infection in the emergency department: an observational study. Crit Care 2004; 8: R12-R20.
- Gaini S, Koldkjaer OG, Pedersen C, et al. Procalcitonin, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in community-acquired infections and sepsis: a prospective study. Crit Care 2006; 10: R53.
- Hausfater P, Garric S, Ayed SB, et al. Usefulness of procalcitonin as a marker of systemic infection in emergency department patients: a prospective study. Clin Infect Dis 2002; 34: 895-901.
- Kofoed K, Andersen O, Kronborg G, el al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care 2007; 11: R38.
- Chirouze C, Schuhmacher H, Rabaud C, et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis 2002; 35: 156-61.
- Ugarte H, Silva E, Mercan D, et al. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med 1999; 27: 498-504.
- Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 2004; 363: 600-07.
- Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008 Oct 13; 168: 2000-07.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007; 131: 9-19.
- Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol 2006; 7: 1266-73.
- Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 2000; 164: 4991-95.
- Bouchon A, Facchetti F, Weigand MA, et al. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001; 410: 1103-07.
- Schenk M, Bouchon A, Seibold F, et al. TREM-1--expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest 2007; 117: 3097-3106.
- Murakami Y, Akahoshi T, Hayashi I, et al. Induction of triggering receptor expressed on myeloid cells 1 in murine resident peritoneal macrophages by monosodium urate monohydrate crystals. Arthritis Rheum 2006; 54: 455-62.
- Gibot S, Kolopp-Sarda MN, Bene MC, et al. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med 2004; 141: 9-15.
- Gibot S, Cravoisy A, Levy B, et al. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med 2004; 350: 451-58.
- Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis 2003; 187 Suppl 2: S397-S401.
- Gomez-Pina V, Soares-Schanoski A, Rodriguez-Rojas A, et al. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol 2007; 179: 4065-73.
- Gibot S, Cravoisy A, Kolopp-Sarda MN, et al. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med 2005; 33: 792-96.
- Phua J, Koay ES, Zhang DH, et al. Soluble triggering receptor expressed on myeloid cells-1 in acute respiratory infections. Eur Respir J 2006; 28: 695-702.
- Tzivras M, Koussoulas V, Giamarellos-Bourboulis EJ, et al. Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease. World J Gastroenterol 2006; 12: 3416-19.
- Adib-Conquy M, Monchi M, Goulenok C, et al. Increased plasma levels of soluble triggering receptor expressed on myeloid cells 1 and procalcitonin after cardiac surgery and cardiac arrest without infection. Shock 2007; 28: 406-10.
- Giamarellos-Bourboulis EJ, Zakynthinos S, Baziaka F, et al. Soluble triggering receptor expressed on myeloid cells 1 as an anti-inflammatory mediator in sepsis. Intensive Care Med 2006; 32: 237-43.
- Bopp C, Hofer S, Bouchon A, el at. Soluble TREM-1 is not suitable for distinguishing between systemic inflammatory response syndrome and sepsis survivors and nonsurvivors in the early stage of acute inflammation. Eur J Anaesthesiol 2009 Mar 20 [Epub ahead of print].
- Jiyong J, Tiancha H, Wei C, et al. Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in bacterial infection: a meta-analysis. Intensive Care Med 2009; 35: 587-95.
- Martins PS, Brunialti MK, da Luz FM, et al. Bacterial recognition and induced cell activation in sepsis. Endocr Metab Immune Disord Drug Targets 2006; 6: 183-91.
- Roger T, David J, Glauser MP, et al. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 2001; 414: 920-24.
- Goldie AS, Fearon KC, Ross JA, et al. Natural cytokine antagonists and endogenous antiendotoxin core antibodies in sepsis syndrome. The Sepsis Intervention Group. JAMA 1995; 274: 172-77.
- Opal SM, Cross AS. Clinical trials for severe sepsis. Past failures, and future hopes. Infect Dis Clin North Am 1999; 13: 285-97, vii.
- Abraham E. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med 1999; 25: 556-66.
- Oda S, Hirasawa H, Shiga H, et al. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine 2005; 29: 169-75.
- Chiesa C, Pellegrini G, Panero A, et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem 2003; 49: 60-68.
- Ventetuolo CE, Levy MM. Biomarkers: diagnosis and risk assessment in sepsis. Clin Chest Med 2008; 29: 591-603.
- Harbarth S, Holeckova K, Froidevaux C, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med 2001; 164: 396-402.
- Carrigan SD, Scott G, Tabrizian M. Toward resolving the challenges of sepsis diagnosis. Clin Chem 2004; 50: 1301-14.
- Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med 2009; 37: 96-104.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








