Printed from acutecaretesting.org
December 2000
Blood gases, acid-base balance, muscle mass and exercise capacity at the upper tolerable limit for humans of acute and chronic hypoxia
INTRODUCTION
An altitude of 5,000 metres above sea level (m.a.s.l) or just above is on the limit of what can be sustained by humans at an acute ascent as well as for more permanent living. Gas exchange and the maintenance of an acceptable acid-base balance are the most immediate functions that determine whether the level of hypoxia is tolerable [1,2].
In a longer perspective the critical issue is whether catabolic and anabolic reactions in cells and tissues can be kept in balance to secure a sufficient respiration, metabolism and protein synthesis. Substrate metabolism and protein metabolism of the cells also undergo adaptive responses to chronic hypoxia, but they do not attract as much attention as the more obvious and direct life-threatening condition related to lack of O2 and an alkaline pH.
Although the changes in cell metabolism are subtle, they will ultimately and over time set the limit for how high and for how long a human being can live at altitude. This became apparent already to some of the first researchers who explored the adaptability of humans to hypoxia.
When Dr. Fillippi returned from the Duke of the Abruzzi’s expedition to the Himalayas in 1911 (quoted from [3]), he wrote:
“The Duke’s expedition offers the clearest proof that men [raised at sea level] can live for extended periods of time, in possession of healthy functional activity of all their organs, at an atmospheric pressure little more than half of normal.
Twelve Europeans and fifteen coolies lived for about two months at above 17,000 feet of altitude, working regularly, and not showing a single case of illness, even of the most fleeting character, attributable to mountain sickness. ....Moreover, rarefaction of the air is not incompatible with mountaineering work, if this is done very slowly and methodically. ....None the less, the experience of the expedition was not one of absolute immunity.
The atmosphere of these heights did work some evil effect, revealing itself only gradually, after several weeks of life above 17,000 feet, in a slow decrease of appetite and consequent lack of nourishment, without, however, any disturbance of the digestive faculties. ....Of course, in the long run, this insufficient nourishment would cause a lowering of vitality, loss of flesh, and a certain amount of anaemia.
However, the process is so slow that we were still at the end of two months in condition to make long marches without experiencing excessive fatigue.”
In this article we will briefly present data on how bodily functions, crucial for survival in a short and longer perspective adjust when abruptly exposed to severe hypoxia.
This acute condition will be compared with the situation where the body has been allowed to more gradually acclimatize to the same degree of hypoxia during a period that is long enough for a lowlander to become almost fully acclimatized.
The variables that will be reported upon are blood gases and acid-base balance both at rest and during exercise, body weight, muscle size, maximal oxygen uptake, and peak power output.
SUBJECTS AND METHODS
The 16 Danish students (10 males and 6 females), all born and raised at sea level (s.l.) were studied at s.l., at acute exposure to the equivalent of 5,260 m.a.s.l. (= ~400 mmHg) by breathing 10 % O2 in N2, as well as at two time points (5 and 9 weeks) at altitude.
Body weight was measured to the nearest 0.5 kg at each test. Cross-sections of the thigh was obtained from multiple (28) magnetic resonance images (MRI; Fig. 1) taken before departure and within 2-3 days upon return to s.l. (note that during this period at s.l. the subjects stayed in a low-pressure chamber almost 24 hours a day).
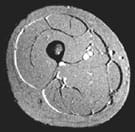 |
FIG. 1: Magnetic resonance cross section image of the thigh with an indication of the location of the various tissues. |
The exercise was performed on a Monark bicycle ergometer. To obtain the maximal oxygen uptake an incremental test was applied with elevations of 20-40 Watts every second minute until exhaustion started at ~100 Watts.
Oxygen uptake was measured continuously during the exercise with Medical Graphics equipment and so was the ECG. Blood gases were determined on blood from the femoral artery with the use of a Radiometer ABL5.
RESULTS WITH COMMENTS
Body weight and muscle mass
The mean body weight of the whole group was 74.5 (53.9-91.1) kg before going to altitude, and it was reduced by 9.6 % to 67.3 (50.0-79.0) kg after the 9 weeks at altitude.
All subjects lost weight but the variation was from a couple to close to 15 kg. MRI data revealed that the lean area was reduced by an average of 9.1 % whereas the fat area was unchanged. The muscle-size changes reflected those observed in body weight. No apparent gender differences were observed.
These data indicate that a dominant portion of the reduction in body weight or at least 50 % under the present conditions was due to a reduction in muscle mass. It is not validated, but most likely that the muscle size of the upper body shrunk to a similar extent as the leg muscles or more.
This is proposed as the muscles of the torso and arms were used less during the stay at altitude. The subjects were extremely active, trekking long distances in the mountains almost daily, but lifting and carrying loads were minimal.
Blood gases and acid-base balance
At rest the effect of the quite severe hypoxia resulted in a drop in the oxygen tension of the arterial blood, pO2(aB), to 47 mmHg, and an arterial oxygen saturation, sO2(aB) of 82 % (Table I).
Concomitantly pH rose to 7.53 due to the pronounced elevation (> 50 %) in the pulmonary ventilation. The time of acute hypoxia at rest was quite short (~5 min) as several subjects experienced dizziness.
|
pO2
|
sO2
|
pH |
||||
|
Acu-
|
Chro-
|
Acu-
|
Chro-
|
Acu-
|
Chro-
|
|
|
Rest
|
47
|
49
|
82
|
85
|
7.53
|
7.47
|
|
pCO2
|
HCO3-
|
|||
|
Acu-
|
Chro-
|
Acu-
|
Chro-
|
|
|
Rest
|
27
|
22
|
20
|
16
|
TABLE I: Arterial blood variables at rest, at a given submaximal exercise intensity, and at short-term (< 3 min) exhaustive exercise in lowlanders acutely exposed to ~5,260 m.a.s.l. and after 9 weeks of acclimatization. Mean values are given for n = 8-9. The variation in response between subjects was very small, giving extremely small SDs.
Indeed, some of the subjects almost fainted, having a pO2(aB) of less than 30 mmHg, a pCO2 of less than 20 mmHg, and a pH of close to 7.60. During exercise in the acute situation, pO2(aB) dropped to close to 30 mmHg with as low an sO2(aB) as ~65 %.
The acidification of the blood, primarily due to lactate accumulation, caused the pH to be reduced to 7.37 (Table I). With acclimatization, pH at rest was lowered only slightly with similar values observed during the exercises at acute exposure to the altitude. On the other hand, the oxygenation of the blood in chronic hypoxia, being similar to acute hypoxia at rest, improved markedly during the exercise with pO2(aB) being 45 mmHg and sO2(aB) ~73 % at exhaustion.
The pCO2(aB) at rest was as low as 27 mmHg in acute hypoxia, being lowered to 22 mmHg in chronic hypoxia. With exercise a further reduction was observed, reaching 25 and 20 mmHg, respectively, at exhaustion. The corresponding bicarbonate values were 20 and 16 mmol·L-1 at rest in acute and chronic hypoxia, respectively with a 5-mmol further reduction at peak exercise in both conditions.
No gender differences were observed, either for the blood gases or for the acid-base condition.
Maximal oxygen uptake and related variables
The mean value for the maximal oxygen uptake was 4.3 L·min-1 at s.l. At acute hypoxia it was reduced to 2.7 L·min-1 with only a very minor elevation occurring during the acclimatization period (Table II). Thus, after 5 weeks at 5,260 m.a.s.l., V.O2(max) was 2.8 L·min-1; a value that did not change further.
This is at a first glance quite surprising as the oxygen-carrying capacity ([Hb]) of the blood was elevated at rest during the acclimatization period from 143 to 187 g·L-1 (Table II).
|
Sea
|
5,260 m.a.s.l
|
Chronic
|
|
|
Maximal oxygen uptake,
|
4.3 |
2.7 |
2.8 |
|
Hemoglobin (rest),
|
143 |
143 |
187 |
|
sO2, % |
96 |
62 |
71 |
|
ctO2(a), mL·L-1 |
184 |
119 |
178 |
|
Heart ratepeak, bpm |
194 |
187 |
146 |
|
Oxygen delivery,
|
4.9 |
3.2 |
3.4 |
TABLE II: Some variables studied at sea level and at ~5,260 m.a.s.l. in lowlanders (n = 8-16), both acutely and after a 9-week acclimatization period. Note that all values are at peak exercise except for the hemoglobin concentration which is the resting value. Hemoconcentration brought [Hb] up with ~10 g·L-1.
At the same time, arterial oxygen saturation increased in the hypoxic condition from 62 to 71 %, rendering an arterial O2 content of 119 ml·L-1 in acute hypoxia and 178 ml·L-1 after 9 weeks at Chacaltaya (Table II).
The latter value is very similar to s.l. values. What is counteracting an improvement of systemic oxygen delivery is the pronounced drop in maximal heart rate, being 194 beats per minute (bpm) at s.l. but only 156 after 5 and 146 bpm after 9 weeks of chronic hypoxia.
The effect of the hypoxia on V.O2(max) is reflected in a similar drop in peak power of 350 Watts at s.l. and just below 250 Watts at 5,260 m.a.s.l. with only a small improvement of 10-20 Watts during the stay at this altitude.
The pattern of adaptation was gender unspecific.
DISCUSSION
The drop in aerobic exercise capacity observed in the present study was ~65 % of s.l. values, which is the anticipated value based on the literature data [1], see Fig. 2.
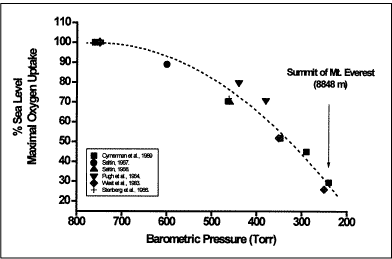
FIG. 2: Maximal oxygen uptake in percent of sea level at various altitudes. Data from the literature as summarized in [1].
The improvement over time at altitude was only very minor in spite of an acclimatization, which proceeded well as indicated not only by the subjects’ perception of well-being, but also by all studied variables.
The very pronounced reduction in pO2(aB) and sO2(aB) as well as the elevation in arterial pH at acute hypoxia were markedly improved by the long acclimatization period, although these variables were still far from s.l. values. Of note is the fact that pH was as high as 7.47 at the end of the stay at Chacaltaya.
It could be that some further improvements could have been accomplished with an even longer stay, but most likely it indicates that blood pH will remain slightly alkaline at this altitude.
The reduction in maximal heart rate at peak exercise that is a well-documented part of the acclimatization process should be looked upon as beneficial to an optimal function in severe hypoxia [4-7].
It is true that systemic oxygen delivery could possibly have been maintained at a slightly higher level if the reduction in HRmax did not occur, and thus V.O2(max) would have been correspondingly higher.
Of note, however, is that by reducing peak cardiac output, mean transit time (MTT) for the red cells passing through the lungs becomes longer. This could be critical and part of the explanation why sO2(aB) is elevated by 10 % while at altitude. This increase occurs in spite of a ~50 % increase in the oxygen-carrying capacity of the blood.
Estimating the MTT at peak exercise load from the measured cardiac output [8,9] at s.l. (23.0 L·min-1) and during acute (21.7 L·min-1) and chronic (16.7 L·min-1) hypoxia, the corresponding MTTs are 1.2, 1.3, and 1.7 seconds, respectively.
This crucial longer passage time of 0.5 s thus allows for a better oxygenation of the blood and it is mediated by the elevated vagal tone to the pacemaker of the heart [5,9,10]. What is unknown, however, is what is sensed and where and how this message is mediated to the autonomic nervous system. There is one more finding worth highlighting in regard to the cardiovascular response to exercise in acute and chronic hypoxia.
The percentage of the cardiac output directed to the exercising limb was ~80 % at acute exposure to hypoxia, but was reduced to ~70 % in the chronic situation [8,9]. This further contributed to V.O2(max) and peak power output not being much improved with the acclimatization period.
Instead, other tissues and organs of the body were better provided for in regard to O2, which probably explains why the subjects, although completely exhausted in all trials, felt more like at s.l. when tested at the end compared with the very early phase of exposure to altitude.
In regard to the change in body weight it is of note that food supply was ample throughout the time at altitude and almost daily, fresh fruit and vegetables were picked up in La Paz.
Claims have been made that body weight can be maintained at very high altitude if a good food supply is available [11]. The present study and several other in the literature point at it being the lean tissue (and thus muscle mass/contractile proteins) that is affected the most.
This is the case not only at extreme altitude, but also at considerably lower altitudes as evidenced by the mean muscle-fiber size determined in muscle tissue obtained with needle biopsies from extremity muscles [12], Table III.
|
Altitude
|
Duration
|
Physical
|
Mean fiber
|
|
> 5,200 |
12 |
High |
5.8 4.7 |
|
~3,700 |
24 |
Low |
4.8 4.2 |
|
~2,700 |
2 |
Very high |
5.8 5.4 |
|
~2,100 |
4
|
Very high
|
5.9 5.5
|
TABLE III: Muscle-fiber sizes (vastus lateralis) in groups of sea-level residents before and after staying at various altitudes for different periods of time (data from [12]). Physical activity level at altitude is included as well.
At present there is no explanation for this selectivity of body tissue being affected the most. At 5,260 m.a.s.l. appetite can be down, but a caloric deficit by itself should have a more general effect on most tissues and organs, not least on the fat pad. Thus, the hypoxia per se appears to induce a shift in the balance in regard to protein synthesis/degradation for contractile proteins.
This could be an integrated part of the general adaptation, which takes place with exposure to severe chronic hypoxia. However, it could also be a quite specific adaptation for one special functional improvement. Skeletal muscle-capillary density is elevated at altitude. This is not because capillaries proliferate in the hypoxic condition. It is because the skeletal muscle-fiber cross-sectional area becomes reduced (Table III [12].
This should be viewed in the light of a crucial role of the diffusion distances in skeletal muscle. One possibility to reduce the distance to the centre of the fiber is to reduce its size. Indeed, based on studies of dogs that were exposed to extreme altitude it has been proposed that skeletal muscle fibers at altitude change their shape from being quite circular to being more elliptic or even rectangular [13].
It seems appropriate to end this article by returning to the quote by Dr. Fillippi in the Introduction. It is true that at very high altitude subtle changes occur in the cells of tissues and organs, which make it impossible to survive there forever.
This is due to the fact that the protein turnover cannot be appropriately regulated with key proteins slowly vanishing as hinted at by Dr. Fillippi already in 1911. Whether this is the only disturbance of homeostasis that sets a limit for prolonged living above ~5,000 m.a.s.l. is uncertain. An alkaline pH, although most likely “only” in blood, could be added as well.
Authors
Bengt Saltin, Professor, Dr. Med.
Director
The Copenhagen Muscle Research Centre
Rigshospitalet (University Hospital)
Section 7652
Blegdamsvej 9
DK-2100 Copenhagen Ø
Denmark
Hans Søndergaard
The Copenhagen Muscle Research Centre
Rigshospitalet (University Hospital)
Copenhagen
Denmark
Morten Zacho
The Copenhagen Muscle Research Centre
Rigshospitalet (University Hospital)
Copenhagen
Denmark
Gerrit van Hall
The Copenhagen Muscle Research Centre
Rigshospitalet (University Hospital)
Copenhagen
Denmark
José A.L. Calbet
Departamento de Educación Física
Campus Universitario de Tafira
Las Palmas de Gran Canaria
Canary Islands
Spain
References+ View more
- Roach RC. Cardiovascular regulation during hypoxia. In: Exercise and Circulation in Health and Disease. Saltin B, Boushel R, Secher N, Mitchell J, eds. Champaign, Ill.: Human Kinetics 2000: 177-94.
- Sutton JR, Reeves JT, Wagner PD, et al. Operation Everest II. Oxygen transport during exercise at extreme simulated altitude. J Appl Physiol 1988; 64: 1309-21.
- Douglas CG, Haldane JS, Henderson Y, Schneider EC. Physiological observations made on Pike’s Peak, Colorado. Phil Trans Roy Soc London 1913; 203: 219-318.
- Christensen EH, Forbes WH. Der Kreislauf in grossen Höhen. Skand Arch Physiol 1937; 76: 75-89.
- Hartley LH, Vogel JA, Cruz JC. Reduction of maximal exercise heart rate at altitude and its reversal with atropine. J Appl Physiol 1974; 36: 362-65.
- Pugh LG. Cardiac output in muscular exercise at 5800 m (19,000 ft). J Appl Physiol 1964; 19: 441-47.
- Saltin B, Grover RF, Blomqvist CG, Hartley LH, Johnson RL. Maximal oxygen uptake and cardiac output after 2 weeks at 4300 m. J Appl Physiol 1968; 25: 400-9.
- Calbet JAL, Boushel R, Rådegran G, Søndergaard H, Saltin B. Cardiovascular response to exercise with severe acute hypoxia. J Physiol 2000; submitted.
- Boushel RC, Rådegran G, Calbet JAL, Søndergaard H, Wagner PD, Saltin B. Parasympathetic neural activity accounts for the lowering of exercise heart rate at high altitude. Circulation 2000; submitted.
- Savard GK, Areskog NH, Saltin B. Cardiovascular response to exercise in humans following acclimatisation to extreme altitude. Acta Physio Scand 1995; 154: 499-509.
- Butterfield GE, Gates J, Fleming S, Brooks GA, Sutton JR, Reeves JT. Increased energy intake minimizes weight loss in men at high altitude. J Appl Physiol 1992; 75: 1741-48.
- Saltin B. Exercise and the environment. Focus on Altitude. Res Quart Exerc Sport 1996; 67, Suppl 3: 1-10.
- Banchero N. Capillary density of skeletal muscle in dogs exposed to simulated altitude. Proc Soc Exp Biol Med 1975; 148: 435-39.
References
- Roach RC. Cardiovascular regulation during hypoxia. In: Exercise and Circulation in Health and Disease. Saltin B, Boushel R, Secher N, Mitchell J, eds. Champaign, Ill.: Human Kinetics 2000: 177-94.
- Sutton JR, Reeves JT, Wagner PD, et al. Operation Everest II. Oxygen transport during exercise at extreme simulated altitude. J Appl Physiol 1988; 64: 1309-21.
- Douglas CG, Haldane JS, Henderson Y, Schneider EC. Physiological observations made on Pike’s Peak, Colorado. Phil Trans Roy Soc London 1913; 203: 219-318.
- Christensen EH, Forbes WH. Der Kreislauf in grossen Höhen. Skand Arch Physiol 1937; 76: 75-89.
- Hartley LH, Vogel JA, Cruz JC. Reduction of maximal exercise heart rate at altitude and its reversal with atropine. J Appl Physiol 1974; 36: 362-65.
- Pugh LG. Cardiac output in muscular exercise at 5800 m (19,000 ft). J Appl Physiol 1964; 19: 441-47.
- Saltin B, Grover RF, Blomqvist CG, Hartley LH, Johnson RL. Maximal oxygen uptake and cardiac output after 2 weeks at 4300 m. J Appl Physiol 1968; 25: 400-9.
- Calbet JAL, Boushel R, Rådegran G, Søndergaard H, Saltin B. Cardiovascular response to exercise with severe acute hypoxia. J Physiol 2000; submitted.
- Boushel RC, Rådegran G, Calbet JAL, Søndergaard H, Wagner PD, Saltin B. Parasympathetic neural activity accounts for the lowering of exercise heart rate at high altitude. Circulation 2000; submitted.
- Savard GK, Areskog NH, Saltin B. Cardiovascular response to exercise in humans following acclimatisation to extreme altitude. Acta Physio Scand 1995; 154: 499-509.
- Butterfield GE, Gates J, Fleming S, Brooks GA, Sutton JR, Reeves JT. Increased energy intake minimizes weight loss in men at high altitude. J Appl Physiol 1992; 75: 1741-48.
- Saltin B. Exercise and the environment. Focus on Altitude. Res Quart Exerc Sport 1996; 67, Suppl 3: 1-10.
- Banchero N. Capillary density of skeletal muscle in dogs exposed to simulated altitude. Proc Soc Exp Biol Med 1975; 148: 435-39.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar








