Printed from acutecaretesting.org
January 2010
Blood oxygenation and spurious hypoxemia
BLOOD OXYGENATION
All cells require a continuous supply of oxygen and one of the principal life-preserving functions of blood is delivery of oxygen present in inspired air from lungs to tissue cells. An adequate supply of oxygen to tissues depends then on adequate oxygenation of blood.
Oxygen is carried in blood in two forms. Most (around 98 %) is carried bound to hemoglobin, but a very small amount is dissolved in blood plasma. It is this very small amount of dissolved unbound oxygen that determines pO2(a). Although pO2(a) reflects only a very small portion of the total oxygen in arterial blood, it is the major determinant of the amount of oxygen carried by hemoglobin (% hemoglobin saturation, sO2(a)) and therefore the total amount of oxygen carried by blood.
The relationship between partial pressure of oxygen in blood (pO2) and hemoglobin oxygen saturation (sO2), described by the oxygen dissociation curve (Fig. 1), determines that when pO2(a) is within the normal range (10-12 kPa), hemoglobin is almost maximally saturated (sO2(a) > 97 %).
Providing that blood contains a normal amount of hemoglobin, sO2(a) 97 % indicates adequate oxygenation.
However, if pO2(a) is reduced to say less than 8.0 kPa, hemoglobin binds significantly less oxygen (sO2(a) < 90%) and, irrespective of hemoglobin concentration, blood is not well oxygenated. It is evident from examination of the linear portion of the oxygen dissociation curve that only a slight reduction in pO2(a) below 8 kPa is associated with a precipitous fall in sO2(a) and therefore a marked reduction in the oxygen content of blood. Respiratory failure is defined by a pO2(a) of less than 8kPa (<60 mmHg).
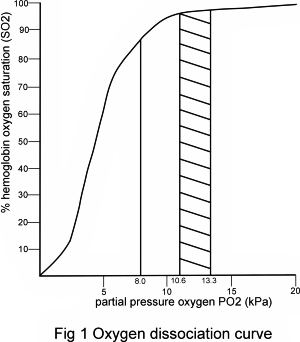
Although adequate oxygenation of blood cannot be guaranteed if pO2(a) or sO2(a) are within their respective reference ranges (there are other considerations, including hemoglobin concentration and presence of dyshemoglobins) [1], reduced pO2(a) and sO2(a) always indicate inadequate blood oxygenation, i.e. hypoxemia.
The more severe the hypoxemia, the greater is the risk of hypoxia (insufficient oxygen to support aerobic metabolism in tissue cells) and consequent cell dysfunction and, ultimately, organ failure.
CAUSES OF REDUCED PO2(A) (HYPOXEMIA)
Oxygen in inspired air diffuses from the alveoli of the lungs to the blood due to the pO2 gradient across the alveolar membrane. Maintenance of normal pO2(a) depends on:
- adequate partial pressure of oxygen in inspired air (pO2(I))
- normal alveolar ventilation (and therefore normal respiratory rate)
- adequate blood perfusion of the alveoli
- match between alveolar ventilation and perfusion (normal V/Q)
Consideration of these four factors allows some understanding of the many causes of reduced pO2(a) (hypoxemia).
At high altitude the attendant low barometric pressure determines that pO2(I) is reduced. Despite normal respiratory and cardiac function, pO2(a) is thus reduced at high altitude.
Reduced global alveolar ventilation is the cause of the reduced pO2(a) that occurs when the respiratory center in the brain is damaged by disease or injury, or depressed by drugs.
Neuromuscular damage affecting the lungs, for example in Guilian Barre syndrome, likewise reduces global alveolar ventilation, leading to reduced pO2(a). A local mismatch between alveolar ventilation and alveolar perfusion causes the reduced pO2(a) that occurs in respiratory infection (pneumonia), chronic obstructive airways disease and pulmonary edema.
The alveoli are well perfused but some alveoli are damaged/congested, thereby reducing local ventilation. Inadequate local alveolar perfusion due to thrombus formation accounts for the hypoxemia that occurs in those suffering pulmonary embolus.
Of particular significance for this article is the hypoxemia and associated respiratory failure that can complicate the course of leukemia and other hematological malignancies.
The etiology of respiratory failure in the context of hematological malignancy varies [2,3] and may be due to infection (pneumonia) consequent on the reduced immunity that the disease and its treatment provokes, or have a non-infectious cause.
Those with extremely high white-cell counts, for example, are at high risk of a syndrome called leukostasis in which white cells aggregate in the microvasculature, inducing endothelium-mediated damage to organs, predominantly the brain and lungs [4].
Respiratory failure is a common consequence of leukostasis. Whether the result of infection or a non-infectious cause such as leukostasis, respiratory failure is a not uncommon complication of hematological malignant disease. For this reason many patients suffering leukemia or related hematological malignant disease may be submitted for arterial blood gas analysis and measurement of pO2(a).
The finding of reduced pO2(a) in these patients particularly, must be interpreted with care. It may reflect the true pO2(a) of arterial blood, allowing a correct diagnosis of hypoxemia and, if sufficiently reduced, respiratory failure. However, it may alternatively be falsely low in which case the correct diagnosis is spurious hypoxemia or pseudohypoxemia.
IN VITRO METABOLISM - THE CAUSE OF SPURIOUS HYPOXEMIA
Spurious hypoxemia arises due to the continuing metabolism of the blood cells after blood has been sampled (i.e. in vitro metabolism). Like all living tissues blood cells continue to metabolize glucose in vitro and this metabolism is associated with consumption of oxygen and production of carbon dioxide.
The significance of this continued in vitro metabolism for the accuracy of blood gas analysis was first quantified in the early 1960s [5,6].
This work determined that measured pO2(a) is affected by a number of factors including: the temperature at which the blood is stored; the time interval between collection and measurement; and the number of oxygen-consuming blood cells (white cells, platelets and reticulocytes) contained in the sample.
Mature erythrocytes contribute little to the total in vitro oxygen consumption because these cells lack mitochondria and metabolize glucose by anaerobic glycolysis. The rate of in vitro oxygen consumption was found to be proportional to white-blood-cell count, platelet count and reticulocyte count.
These and related findings provided the rationale for the development of the now familiar protocol for processing arterial blood samples prior to analysis.
To minimize the effect of in vitro oxygen consumption and thereby ensure that the measured pO2(a) most accurately reflects in vivo pO2(a), samples must be analyzed immediately, or at least within 30 minutes.
Storage of specimens in iced water also reduces in vitro metabolism, although caution in the use of this strategy is recommended if the blood is sampled to plastic syringes.
These routine precautions are not sufficient to prevent significant in vitro oxygen consumption if the sample contains an extremely high number of white cells or platelets, and it is in the context of hyperleucocytosis (white-blood-cell count > 100 × 109/L) or extreme thrombocytosis (platelet count > 2000 × 109/L) that spurious hypoxemia occurs.
These extreme blood counts are only really encountered in patients suffering leukemia and other hematological malignant disease so that the problem of spurious hypoxemia is confined to this patient group. Since immature leukemic (blast) cells consume more oxygen than normal white cells, it is not only the number of white cells but also their immaturity that contribute to spurious hypoxemia in leukemic patients.
DEMONSTRATING THE LINK BETWEEN HYPERLEUCOCYTOSIS AND SPURIOUS HYPOXEMIA
Spurious hypoxemia was first described in 1979 [7,8] by physicians who noted that some patients suffering leukemia or thrombocytosis had reduced pO2(a), despite no clinical evidence of disease that might compromise blood oxygenation, or symptoms of reduced oxygenation.
These patients all had an extremely high white-cell count or platelet count, and pO2(a) spontaneously reverted to normal when the white-cell count or platelet count was reduced by chemotherapy.
An early study of two such patients by Fox et al [9] confirmed and quantified the link between hyperleucocytosis and spurious hypoxemia that clinical observation suggested. The first patient was a 27-year-old male with chronic myeloid leukemia.
At the time of the study, 18 months after diagnosis, his white-blood-cell count was 276 × 109/L. His measured pO2(a) when breathing room air was 4.0 kPa (30 mmHg). Despite this evidence of life-threatening hypoxemia he had no pulmonary symptoms, and nothing abnormal was detected on chest X-ray film.
The second patient was a 74-year-old male with chronic lymphocytic leukemia. His white-blood-cell count was 360 × 109/L. He, too, had no pulmonary symptoms and a clear chest film, but his measured pO2(a) was 7.0 kPa (53 mmHg), indicating marked hypoxemia and respiratory failure.
For the study, arterial blood from each patient and a healthy control was tonometered with a gas mixture containing 20.6 % oxygen (partial pressure of the order 20 kPa or 150 mmHg).
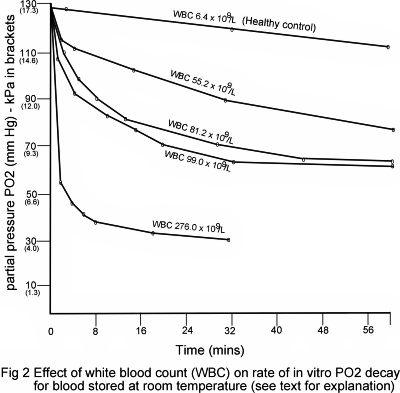
After 30 minutes, equilibration blood was withdrawn from the tonometer to a glass syringe and pO2(a) measured immediately, and at intervals during the following hour, to monitor the in vitro decay in pO2(a). During this measuring hour blood was stored in the syringe under anaerobic conditions at room temperature (22 °C).
This procedure was repeated on four separate occasions over a 7-day period, during which the patients' white-cell count decreased in response to therapy.
The results relating to the first patient (Fig. 2) demonstrate clearly that the rate of pO2(a) in vitro decay was significantly higher than the control but reduced towards normal as the white-cell count was reduced from 276 × 109/L to 55 × 109/L. Of great significance was the finding that the rate of decay is greatest during the first 2 minutes after sampling.
For blood with a white-cell count of 276 × 109/L, pO2(a) was found to fall by a staggering 9.6 kPa (72 mmHg) during the first 2 minutes after removal from the tonometer.
The group demonstrated that in vitro decay in pO2(a) could be entirely eliminated by addition of potassium cyanide (0.5 mg/1 mL blood) to the blood, prior to tonometry, providing evidence that cellular respiration (oxygen consumption) was responsible for the observed in vitro pO2(a) decay.
In a separate experiment, the effect of lowering the temperature was tested by storing blood on ice during the measuring hour. This blunted, but did not eliminate in vitro pO2(a) decay; a finding that confirms that placing arterial blood samples on ice does not entirely eliminate spurious hypoxemia [10,11] as some have suggested [8].
This blunted response can be accounted for by the observation that the rate of pO2(a) decay is greatest in the minutes immediately after sampling, during the time that temperature equilibration with iced water is occurring.
IDENTIFYING SPURIOUS HYPOXEMIA
A number of strategies have been proposed for identifying spurious hypoxemia. These have included the use of an intra-arterial continuous blood gas monitoring device [12]; addition of sodium cyanide or sodium fluoride to arterial blood prior to blood gas analysis [9,13] and the use of arterial plasma rather than arterial blood to measure pO2(a) [14].
The simplest and most widely used strategy is to employ pulse oximetry [11,15,16]. Pulse oximetry provides an alternative to arterial blood gas analysis for assessing blood oxygenation that depends on non-invasive measurement of capillary oxygen saturation.
Oxygen saturation as measured by pulse oximetry (SpO2) approximates closely to sO2(a) . In cases of spurious hypoxemia, because pO2(a) is falsely low, the calculated value for sO2(a), generated simultaneously during arterial blood gas analysis, is also falsely low.
However, sO2(a), as measured by pulse oximetry (SpO2) accurately reflects in vivo sO2(a). Thus spurious hypoxemia is characterized by a disparity between SpO2 (normal) and pO2(a) and sO2(a) (both markedly reduced).
Of course it is entirely conceivable that the conditions that give rise to spurious hypoxemia (extreme hyperleucocytosis/thrombocytosis) may be present in a patient who also has a problem that compromises gas exchange, and therefore an actual reduction in pO2(a). In other words, spurious hypoxemia and true hypoxemia can co-exist.
In such cases SpO2 will be reduced but pO2(a) and sO2(a) will be reduced to a much greater extent; the marked disparity between SpO2 and pO2(a)/sO2(a) will still be evident.
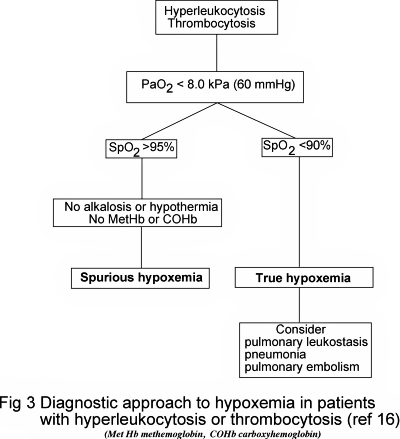
Whilst recommending the use of pulse oximetry to identify spurious hypoxemia Lele A et al [16] rightly caution that SpO2 can be falsely raised (thereby masking true hypoxemia) if the blood contains an abnormally high concentration of dyshemoglobins (carboxyhemoglobin, COHb; methemoglobin, MetHb).
In addition, severe metabolic alkalosis or hypothermia shifts the oxygen dissociation curve to the right so that sO2(a) (and therefore SpO2) will be higher for any given pO2(a). They provide a useful algorithm (Fig. 3) for the investigation of suspected spurious hypoxemia that takes account of these limitations of pulse oximetry.
ILLUSTRATIVE CASE HISTORIES
Case 1 - Spurious hypoxemia leads to unnecessary intervention
This case [17] concerns a 34-year-old woman with acute lymphoblastic leukemia (ALL) who was admitted to hospital 10 months after diagnosis because she had become short of breath during the previous 10 days.
The breathlessness had been accompanied by sore throat, productive cough and fatigue. Respiratory rate was increased (36/min).
Full blood count on admission revealed anemia (Hb 6.9 g/L) and hyperleukocytosis (white-cell-count 191 × 109/L, 77 % lymphoblasts). Chest X-ray was normal. A diagnosis of bronchitis was made and intravenous antibiotics were prescribed, eliciting improvement.
On the second day, however, she again complained of cough and breathlessness and this, despite no change on physical examination, no cyanosis and a second normal chest X-ray, provoked a request for arterial blood gas analysis.
This revealed a pO2(a) of 4.4 kPa (33 mmHg) and a calculated sO2(a) of 78.7 %. This evidence of severe hypoxemia accompanying dyspnea prompted major intervention. The patient was intubated and placed on a mechanical ventilator.
However, spurious hypoxemia was emerging as a possible explanation for the apparent disconnect between the documented severe hypoxemia and the patient’s relatively mild clinical presentation. Later on the same day she was weaned off the respirator, extubated and treated with oxygen (10 L/min) via a mask.
During oxygen therapy pO2(a) (6.3 kPa) and sO2(a) (79.8 %) remained unaccountably low.
Eventually a diagnosis of spurious hypoxemia, secondary to hyperleucocytosis, was made when pulse oximetry revealed a marked disparity between SpO2 (94 %) and pO2(a) (4.4 and 6.3 kPa) and sO2(a) (78.7 and 79.8 %). A third blood gas sample was taken whilst the patient was still receiving oxygen, but on this occasion the sample was immediately iced and analyzed to minimize in vitro pO2(a) decay. pO2(a) of this sample was 26.3 kPa (197 mmHg) and sO2(a) 100 %.
The patient, who presumably at no time during her hospital stay had actually suffered significant reduction in blood oxygenation, soon recovered from her upper-respiratory-tract infection and was discharged home.
Clearly, mechanical ventilation and oxygen therapy had been administered inappropriately in this case because spurious hypoxemia had gone unrecognized.
Case 2 – Spurious hypoxemia in a patient with normal white-cell count
This case [18] concerns a 72-year-old lady who presented first with complaint of fatigue. Blood count revealed raised hemoglobin, Hb (18.7 g/dL) and hematocrit, Hct (56 %), normal white-cell count (8.3 × 109/L) and very high platelet count (2,168 × 109/L).
Further blood testing to investigate apparent polycythemia (raised Hb Hct) revealed increased red-cell mass and reduced erythropoietin. Examination of a bone-marrow biopsy found abnormalities consistent with a diagnosis of polycythemia vera, a myeloproliferative (malignant) disease. This diagnosis is entirely consistent with initial blood test results.
Arterial blood gas analysis at this time revealed evidence of marked hypoxemia and respiratory failure (pO2(a) 6.2 kPa, 47 mmHg; and sO2(a) 74 %).
Intensive investigation followed to explain this finding. It was supposed that the hypoxemia was genuine and might be the cause of polycythemia (secondary erythrocytosis).
This series of investigations included chest radiography, computed tomography scan of chest, pulmonary function tests, transthoracic bubble-contrast echocardiography and p50 testing for possible high-affinity hemoglobinopathy.
None of this testing revealed any abnormality, and 3 months after initial presentation the patient was referred to a tertiary medical facility for investigation of unexplained marked hypoxemia.
Here initial evaluation included pulse oximetry. The patient’s SpO2 was 98 %, indicating normal blood oxygenation. Simultaneously collected blood gases, however, indicated, as before, severe hypoxemia (pO2(a) 5.3 kPa, 40 mmHg; sO2(a) 73 %).
The disparity between pulse oximetry and blood gas results suggested spurious hypoxemia due to extreme thrombocytosis (platelet count at this time was 2,425 × 109/L). To test this hypothesis, blood gases were repeated, but on this occasion KCN (1 mM final concentration) was added to the sample to eliminate in vitro oxygen consumption.
Results from this adulterated sample were entirely normal (pO2(a) 12.5 kPa, 94 mmHg; and sO2(a) 98 %) and consistent with SpO2 (98 %) recorded during blood sampling. This confirmed spurious hypoxemia.
Treatment for polycythemia vera was immediately instituted and over the following weeks and months hematocrit and platelet counts reduced towards normal as the patient's condition improved.
At 20 months after initiation of treatment, platelet count was 721 × 109/L and blood gases on a sample collected conventionally (i.e. without KCN) at this time returned a normal pO2(a) (11.1 kPa, 84 mmHg) and sO2(a) (97 %) result.
This case history demonstrates that spurious hypoxemia can occur in patients with normal white-cell count if platelet count is very high. It also serves to remind that, if not recognized, spurious hypoxemia can lead to needless investigation and delay in appropriate treatment.
SUMMARY
Spurious hypoxemia is a disparity between measured and actual pO2(a) due to in vitro consumption of oxygen by blood cells (white cells and platelets).
Spurious hypoxemia occurs in those with very high white-cell count (>100 × 109/L or very high platelet count (>1500 × 109/L). Such extreme blood counts are confined to those with hematological malignant disease.
Failure to identify spurious hypoxemia can lead to needless investigation and inappropriate intervention, including mechanical ventilation.
Pulse oximetry provides a simple, reliable way of identifying spurious hypoxemia and the means of monitoring blood oxygenation in those with spurious hypoxemia.
Spurious hypoxemia represents just one of many ways in which hematological conditions give rise to factitious biochemical measurements. The wider topic is the subject of a recent comprehensive review [19].
References1. Prakash ES, Madanmohan. What does one mean by "arterial blood oxygenation?" Adv Physiol Edu 2006; 30: 46-47.
2. Ewig S, Torres A, Riquelme R et al. Pulmonary complications in patients with haematological malignancies treated at a respiratory ICU. Eur Respir J 1998; 12: 116-22.
3. Chaoui D, Legrand O, Roche N et al. Incidence and prognostic value of respiratory events in acute leukemia. Leukemia 2004; 18: 670-75.
4. Porcu P, Cripe L, Ng E et al. Hyperleukocytic leukemias and leukostasis: A review of pathophysiology, clinical presentation and management. Leukemia and Lymphoma 2000; 39: 1-18.
5. Anderson O. Sampling and storing of blood for determination of acid base status. Scand J Clin Lab Invest 1961; 13: 196-204.
6. Lenfant C, Aucutt C. Oxygen uptake and change in carbon dioxide tension in human blood stored at 37oC. J Applied Physiol 1965; 20: 503-08.
7. Chillar RK, Belman MJ. Fictitious hypoxemia associated with extreme leukocytosis. Proc Am Assoc Cancer Res Am Soc Clin Oncol. 1979; 20: 364.
8. Hess C, Nicholls A, Hunt W et al. Pseudohypoxemia secondary to leukemia and thombocytosis. New Eng J Med 1979; 301: 361-63.
9. Fox M, Brody J, Weintraub L et al. Leucocyte larceny: a cause of spurious hypoxemia. Am J Med 1979; 67: 742-46.
10. Chillar R, Belman M, Farbsterin M et al. Explanation of apparent hypoxemia associated with extreme leukocytosis: leukocytic oxygen consumption. Blood 1980; 55: 922-24.
11. Loke J, Duffy T. Normal arterial oxygen saturation with ear oximeter in patients with leukemia and leukocytosis. Cancer 1984; 53: 1767-69.
12. Mizcock B, Franklin C, Lindesmith P et al. Confirmation of spurious hypoxemia using continuous blood gas analysis in a patient with chronic myelogenous leukemia. Leukemia Res 1995; 19: 1001-04.
13. Schmaier A. Pseudohypoxemia due to leukemia and thrombocytosis. New Eng J Med 1980; 302: 584.
14. Charan NB, Marks M, Carvalho P. Use of plasma for arterial blood gas analysis in leukemia. Chest 1994; 105: 954-55.
15. Mutlu G, Sznajder J. Pseudohypoxemia: interpretation of discrepancies between SaO2 and SpO2. Tuberkuloz ve Toraks Dergisi 2005; 53: 185-89.
16. Lele A, Mirski M, Stevens R. Spurious hypoxemia. Crit Care Med 2005; 33: 1854-56.
17. Charoenratanakul S, Loasuthi K. Pseudohypoxemia in a patient with acute leukemia. Thorax 1997; 52: 394-95.
18. Mehta A, Lichtin A, Vigg A et al. Platelet larceny: spurious hypoxaemia due to extreme thrombocytosis. Eur Respir J 2008; 31: 469-72.
19. Dala B, Brigden M. Factitious biochemical measurements resulting from hematologic conditions. Am J Clin Pathol 2009; 131: 195-204.
References1. Prakash ES, Madanmohan. What does one mean by "arterial blood oxygenation?" Adv Physiol Edu 2006; 30: 46-47.
2. Ewig S, Torres A, Riquelme R et al. Pulmonary complications in patients with haematological malignancies treated at a respiratory ICU. Eur Respir J 1998; 12: 116-22.
3. Chaoui D, Legrand O, Roche N et al. Incidence and prognostic value of respiratory events in acute leukemia. Leukemia 2004; 18: 670-75.
4. Porcu P, Cripe L, Ng E et al. Hyperleukocytic leukemias and leukostasis: A review of pathophysiology, clinical presentation and management. Leukemia and Lymphoma 2000; 39: 1-18.
5. Anderson O. Sampling and storing of blood for determination of acid base status. Scand J Clin Lab Invest 1961; 13: 196-204.
6. Lenfant C, Aucutt C. Oxygen uptake and change in carbon dioxide tension in human blood stored at 37oC. J Applied Physiol 1965; 20: 503-08.
7. Chillar RK, Belman MJ. Fictitious hypoxemia associated with extreme leukocytosis. Proc Am Assoc Cancer Res Am Soc Clin Oncol. 1979; 20: 364.
8. Hess C, Nicholls A, Hunt W et al. Pseudohypoxemia secondary to leukemia and thombocytosis. New Eng J Med 1979; 301: 361-63.
9. Fox M, Brody J, Weintraub L et al. Leucocyte larceny: a cause of spurious hypoxemia. Am J Med 1979; 67: 742-46.
10. Chillar R, Belman M, Farbsterin M et al. Explanation of apparent hypoxemia associated with extreme leukocytosis: leukocytic oxygen consumption. Blood 1980; 55: 922-24.
11. Loke J, Duffy T. Normal arterial oxygen saturation with ear oximeter in patients with leukemia and leukocytosis. Cancer 1984; 53: 1767-69.
12. Mizcock B, Franklin C, Lindesmith P et al. Confirmation of spurious hypoxemia using continuous blood gas analysis in a patient with chronic myelogenous leukemia. Leukemia Res 1995; 19: 1001-04.
13. Schmaier A. Pseudohypoxemia due to leukemia and thrombocytosis. New Eng J Med 1980; 302: 584.
14. Charan NB, Marks M, Carvalho P. Use of plasma for arterial blood gas analysis in leukemia. Chest 1994; 105: 954-55.
15. Mutlu G, Sznajder J. Pseudohypoxemia: interpretation of discrepancies between SaO2 and SpO2. Tuberkuloz ve Toraks Dergisi 2005; 53: 185-89.
16. Lele A, Mirski M, Stevens R. Spurious hypoxemia. Crit Care Med 2005; 33: 1854-56.
17. Charoenratanakul S, Loasuthi K. Pseudohypoxemia in a patient with acute leukemia. Thorax 1997; 52: 394-95.
18. Mehta A, Lichtin A, Vigg A et al. Platelet larceny: spurious hypoxaemia due to extreme thrombocytosis. Eur Respir J 2008; 31: 469-72.
19. Dala B, Brigden M. Factitious biochemical measurements resulting from hematologic conditions. Am J Clin Pathol 2009; 131: 195-204.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









