Printed from acutecaretesting.org
July 2008
Capillary blood gases - to arterialize or not
Blood gas analyzers measure blood pH and the oxygen and carbon dioxide tensions of blood (pCO2 and pO2).
These measurements along with parameters (bicarbonate, base excess etc) derived by calculation from these measurements allow evaluation of acid-base status and adequacy of ventilation and oxygenation.
Thus blood gas analysis is helpful for assessment and monitoring of patients suffering a range of metabolic disturbances and respiratory diseases both acute and chronic; it is an important component of the physiological monitoring that critically ill patients, particularly those being mechanically ventilated, require.
The ‘gold standard’ sample for blood gas analysis is arterial blood obtained anaerobically via an indwelling arterial catheter (most often sited at the radial artery in adults and the umbilical artery in neonates), or arterial puncture.
In an intensive care setting where patients may require frequent (perhaps two hourly) blood gas testing, arterial catheterization may be justified because it allows not only convenient and painless access to arterial blood but also continuous blood pressure monitoring.
Placing an arterial catheter is however an invasive, painful and technically difficult procedure [1], which is associated with risk of serious complications including systemic infection, hemorrhage, thrombosis and ischemia [2].
Technical and safety considerations determine that for most patients who require blood gas analysis placement of an arterial catheter is either not justified or justified for only a limited period, so that arterial blood is most often sampled by arterial puncture using needle and syringe.
The most usual puncture site is the radial artery in the wrist; alternative sites include the brachial artery in the arm and femoral artery in the groin. Although arterial puncture does not place patients at risk of the serious complications associated with arterial catheterization, it is potentially hazardous and certainly not risk free [3].
Furthermore it is a painful procedure that is reported by patients to be significantly more painful than venous puncture [4]. Specialist training in arterial puncture is essential for patient safety and comfort, and in many countries obtaining arterial blood is the almost exclusive preserve of medically qualified staff.
Capillary blood can be obtained by near painless [5] skin puncture using a lancet or automated incision device that punctures the skin to a depth of just 1mm [6,18]. It is the least invasive and safest blood collecting technique and can be performed by all healthcare personnel after minimal training [9].
The relative simplicity and safety profile of capillary blood sampling and the necessity for only small volumes (100-150 µL) of blood for pH and gas analysis make capillary blood an attractive substitute for arterial blood, particularly among neonates and infants, but also adults.
However the clinical value of capillary blood gas results depends on the extent to which pH, pCO2 and pO2 of capillary blood accurately reflect pH, pCO2 and pO2 of arterial blood.
CAPILLARY AND ARTERIAL BLOOD - SOME THEORETICAL CONSIDERATIONS
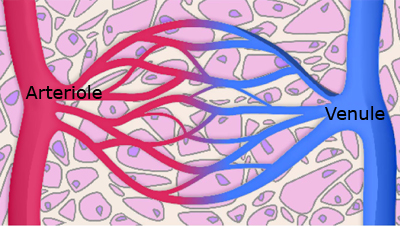
With a diameter of just 8 µm, capillaries are the smallest blood vessel. They are the connection between arterioles (the smallest artery) and venules (the smallest vein) and thus between the arterial and venous sides of the circulatory system.
The capillary network (see FIGURE 1) is the site of nutrient and waste exchange between blood and tissue cells, made possible by the single cell (1 µm) thickness of the capillary wall.
Oxygenated arterial blood arriving via arterioles at the capillary network yields up its oxygen and other essential nutrients to tissue cells as carbon dioxide and other waste products of metabolism are added to blood for transport from tissue cells via venules and the venous system.
As a consequence of these exchanges there is a pH, pCO2 and pO2 gradient across the capillary network (from arteriole to venule), known as the arteriovenous (AV) difference.
Thus for example, the pO2 of blood in arterioles is normally 13 kPa, but following loss of oxygen to tissues only 5 kPa in venules, giving an AV difference for pO2 of approximately 8 kPa [7]. The normal AV differences for pH and pCO2 are of the order 0.02–0.03 pH units and 0.6–0.7 kPa, respectively [8].
FIGURE 1: Capillary Network
| Arterial blood | AV Difference | Venous Blood | |||
| pH | 7.40 | pH | 0.02 | pH | 7.38 |
| pCO2 | 5.3 kPa | pCO2 | 0.7 | pCO2 | 6.0 |
| pO2 | 13.0 kPa | pO2 | 8.0 | pO2 | 5.0 |
Given the anatomical relationship of capillaries to arterioles and venules, it might be supposed that the pH, pCO2 and pO2 of capillary blood would lie roughly midway between arterial and venous values.
That is however not the case because blood obtained by skin puncture is not actually pure capillary blood, but a mixture of blood from punctured arterioles, capillaries and venules (along with a small but variable contribution of interstitial fluid and intracellular fluid from damaged tissue cells) [9].
Due to the relative high pressure on the arterial side of the circulation, this blood mixture contains a relatively greater proportion of blood from the arteriole side of the capillary bed than from the venule side, and thus a ‘capillary’ blood sample obtained by skin puncture approximates closer to arterial blood than venous blood.
This is the theoretical justification for the use of capillary blood as a substitute for arterial blood.
AV difference is clearly a major theoretical determinant of difference between arterial and capillary blood gas values. The greater the AV difference the worse the agreement [10].
By this argument it can be predicted that pO2 (which exhibits a relatively high AV difference) is less likely to show good agreement between capillary and arterial blood than pCO2 and pH (which both, by comparison, have a low AV difference).
Furthermore reduced pO2 (hypoxemia) is associated with reduction in AV difference and hyperoxemia with increased AV difference [7]. Thus there is good theoretical reason to suppose that capillary and arterial blood pO2 will agree more closely if arterial pO2 is reduced than if arterial pO2 is normal or raised.
So long as tissue oxygen consumption and carbon dioxide production remains unchanged, as is the case in the resting state, increasing blood flow through the capillary bed has the effect of reducing AV difference and thereby the difference between arterial and capillary pH, pCO2 and pO2.
This provides the rationale for strategies such as pre-warming the puncture site or treating the puncture site with vasoactive agents prior to capillary blood sampling.
The increased local blood flow that is presumed to occur when these pre-sampling strategies are adopted, theoretically at least, leads to so called ‘arterialization’ of capillary blood and pH, pCO2 and pO2 values that more accurately reflect those of arterial blood.
ARTERIAL AND CAPILLARY BLOOD GAS pH, pCO2 & pO2 - STUDY FINDINGS
Several studies of healthy individuals have defined reference ranges for capillary blood pH, pCO2 and pO2 [11,12]. The results of at least one [11] demonstrate that for healthy adults, sampling ‘arterialized’ capillary blood provides results that are not significantly different from those obtained from arterial blood (see TABLE 1).
Of greater significance are the many more studies conducted over the past 40 years [10,13-27] that have compared blood gas values of simultaneously collected capillary and arterial blood in patients whose clinical condition demands blood gas analysis.
In general they have revealed that whilst capillary blood pH and pCO2 reflects arterial pH and pCO2 sufficiently accurately for clinical purposes, that may not be the case for pO2. Studies in this area have focused exclusively on either pediatric patients (mostly neonates) [13–21] or adult patients [10,21–27].
TABLE 1: Reference ranges for arterial and "arterialised" capillary blood
| Arterial | Capillary | |
|
pH Hydrogen |
7.35-7.45 35-45 |
7.402 (mean only) 37-43 |
| pCO2 (kPa) |
4.7-6.0 | 4.8-6.0 |
| pO2 (kPa) |
10.6-13.3 | 11.2-14.5 |
| Capillary values derived from 103 healthy adults (aged 18-24 yrs) Ref [11] | ||
Studies of pediatric patients
The capillary blood for all studies of neonates and young infants [13–16,18,20] was sampled by heel stab. The method of arterialization was almost exclusively heel warming, usually by immersing the heel in warm water (40–45 ºC) for 5–10 minutes prior to heel stab, or using a warmed surgical plastic device [18].
The rather cumbersome method of histamine iontophoresis was used to arterialize capillary blood of neonates in one early study [15]. Finger stab was the preferred site for sampling capillary blood from children [17,19].
The vast majority of studies reveal clinically acceptable agreement between capillary and arterial pH - a difference of less than 0.05 pH units being considered clinically insignificant [16,17].
In one study [19] of 75 paired samples the mean of capillary pH results was identical to the mean of arterial results and in all other studies the mean difference ranged from 0.001 pH units [14] to 0.02 pH units [20].
One of the larger studies in which 158 paired samples from 41 preterm neonates were compared [16], despite a mean difference of just 0.001 pH units, 24 % of paired samples gave clinically discrepant results (i.e. a difference of > 0.05 pH units).
This however did not detract the authors from the conclusion that capillary blood is a ‘satisfactory’ alternative to arterial blood for measurement of pH. Closer agreement was revealed by a later study [17] of 50 babies and children being cared for in a pediatric intensive care unit.
Here the mean difference between capillary and arterial pH was just 0.009 pH units (95 % limits of agreement ± 0.032) and in no patient was there a difference greater than 0.05 pH units. Johnson et al [18] and Hunt [15] reported no significant difference between capillary and arterial pH of 21 sick neonates (aged from three hours to seven days) and 44 sick babies (aged 3.5 days to 10 weeks).
In common with pH, most studies reveal clinically acceptable agreement between ‘arterialized’ capillary pCO2 and arterial pCO2 - a difference of less than 1 kPa [16] or less than 0.87 kPa [17] being considered clinically insignificant.
All studies revealed the same bias with mean of capillary pCO2 values greater than the mean of arterial pCO2, although this difference was in most studies small, ranging from 0.04 kPa [20] to 0.21 kPa [17].
This last study [17] showed clinically discrepant results (difference greater than 0.87 kPa) in only 2/50 (4 %) paired samples. One of few studies to have revealed poor agreement was that of Hunt [15]. Here the mean difference between capillary and arterial pCO2 was 2.0 kPa.
All studies reveal a bias with regard to pO2 with mean of arterial pO2 values greater than mean of capillary pO2 values. Most studies reveal that this difference is of sufficient magnitude to conclude that there is an unacceptably low level of agreement between capillary and arterial pO2.
McClain et al [16] found a mean pO2 difference of 2.4 kPa, with 84 % of paired samples differing by more than 1 kPa, 56 % differing by more than 2 kPa and 24 % differing by more than 3 ka.
They judge that difference of > 1 kPa has clinical significance. Harrison et al [17] found a mean difference in pO2 between the sample types of 3.3 kPa. In 42/50 (84 %) of paired samples the difference exceeded 0.87 kPa, the difference limit they had set for clinical acceptability.
Bland-Altman plot revealed that the magnitude of the difference between arterial and capillary pO2 depends on arterial pO2. As arterial pO2 increases so too does the difference between capillary and arterial pO2.
Conversely as arterial pO2 decreases the difference decreases. So striking was this effect that Harrison et al found acceptable agreement between capillary and arterial pO2 in all paired samples with arterial pO2 less than < 8 kPa.
This is in agreement with other studies that have included sufficient numbers of severely hypoxemic neonates [16,15].
Arterialization strategies not effective
Several of these studies [15,16,18] tested the effectiveness of strategies aimed at arterializing capillary blood. Hunt et al [15] simultaneously collected arterialized (by histamine iontophoresis) and non-arterialized capillary samples from each study subject for comparison with arterial blood and found no difference in pH, pCO2 and pO2 results for arterialized and non-arterialized capillary samples.
Likewise Johnson et al [18] found that warming babies heels in a plastic molded heating device for on average seven minutes had no arterializing effect; there was no significant difference in pH, pCO2 and pO2 for capillary blood sampled from a warmed heel compared with capillary blood sampled at the same time from the contralateral unwarmed heel.
The greatest arterializing effect of heel warming was found by McLain et al [16], but although mean values for pH, pCO2 and pO2 of warmed heel blood were all slightly closer to mean values for arterial blood than were mean values derived from unwarmed heel blood, on statistical analysis the differences were again found to be insignificant.
These results are in accord with other recent studies [28,29] that have found heel warming has no effect in terms of improved blood flow, an indication of effective arterialization.
A study conducted nearly 50 years ago [30] provides limited evidence that heel warming is effective. This compared capillary with arterial blood pH, pCO2 (but not pO2) in 106 neonates (all less than two weeks old).
In total 149 sample pairs were obtained for comparison. The heel was warmed before collection of capillary blood in 126 instances. For the remaining 23 pairs, capillary blood was collected without prior heel warming.
Superior agreement was observed for both pH and pCO2 for the 126 arterial versus warmed capillary blood pairs compared with the 23 arterial versus unwarmed capillary blood pairs.
The Clinical and Laboratory Standards Institute (CLSI) document H4-A5 on the subject of capillary blood sampling states that: “The need for heel warming is not universal in the literature.
The cited references provide data showing no significant difference of analyte measurement (pH, blood gas, electrolytes) between warming and nonwarming for capillary collection.
Although studies show that prewarming may not be necessary when using a skin incision device, increasing blood flow may be necessary to prevent hemolysis and/or contamination with tissue fluids when using other devices or as a general practice.”
Studies of adult patients
The fingertip or, more commonly, the lower tip of the earlobe are the usual sites of capillary blood sampling in adults, and the most common method of arterialization is application of a vasodilating cream (e.g. Algipan) to the puncture site 5-10 minutes prior to blood sampling.
Of many studies [22–27] that have compared ‘arterialized’ capillary blood gases with arterial blood gases in adults, probably the most informative is a recently published meta-analysis by Gerald Zavorsky and colleagues at McGill University [27].
The database that this group recovered from 29 previously published studies comprised 664 paired samples for comparison of earlobe capillary with arterial blood and 222 paired samples for comparison of fingertip capillary blood with arterial blood. The pH of these 886 paired samples ranged from 6.77–7.74; pCO2 from 1.3–15.1 kPa and pO2 from 2.8–20.6 kPa.
Both fingertip and earlobe capillary pH were found to accurately reflect arterial pH. The mean difference between arterial and earlobe capillary pH was 0.01 ± 0.02 pH units. Regression analysis for this comparison revealed a coefficient of determination (r2) = 0.94 and a residual standard error of 0.025. Very similar results were found for analysis of fingertip capillary versus arterial pairs.
Capillary pCO2 values were also found to be very close to those of arterial blood.
Mean difference between arterial and earlobe capillary blood pCO2 was 0.01kPa ± 0.38. Coefficient of determination (r2) = 0.94; residual standard error 0.4 kPa. Although judged acceptable, agreement between fingertip capillary and arterial pCO2 was not as close as that between earlobe capillary and arterial pCO2.
There was poor agreement between fingertip capillary and arterial pO2. Mean difference was 1.4 ± 2.0 kPa. Coefficient of determination (r2) = 0.48; residual standard error 2.0 kPa. By comparison, better agreement was evident between earlobe capillary and arterial pO2.
Here mean difference was 0.3 ± 0.8 kPa. Correlation of determination (r2) = 0.88; residual standard error 0.8 kPa. There was unequivocal evidence that agreement between capillary (both types) and arterial pO2 improves as arterial pO2 falls.
The authors of this significant study conclude that capillary blood sampled from either the fingertip or earlobe (preferably), accurately reflects arterial pH and pCO2 over a wide range of values. Sampling blood from the earlobe (but never the fingertip) may be an appropriate substitute for arterial pO2 unless precision is required.
The large standard error associated with earlobe capillary pO2 measurement limits its clinical usefulness.
SUMMARY
There is consensus that capillary blood is a clinically acceptable sample alternative to arterial blood if only acid-base parameters (pH and pCO2) are of interest.
Most studies conducted prior to the mid-1990s [5,13,22,23] suggested that capillary blood pO2 reflected arterial pO2 sufficiently accurately for clinical purposes and that capillary blood could justifiably be used as a substitute for arterial blood, not only to assess patient acid-base status, but also oxygenation status.
The results of recent studies [10,18-21,24-27] have challenged that view and the relatively poor agreement between capillary and arterial pO2, most marked if pO2 is raised and least marked if pO2 is low, revealed by these studies, suggest that capillary pO2 results have limited clinical value and should be interpreted with caution.
Capillary blood sampled from the fingertip is particularly unsuited for assessment of oxygenation status [27]. There is really no substitute for arterial blood if accuracy of pO2 measurement is important, for example, for the prescription of long-term oxygen therapy [26].
There is evidence from several studies to suggest that the ritual of warming the heel of babies prior to sampling capillary blood is not effective in ‘arterializing’ capillary blood. There is little, if any, contrary evidence to suggest it is effective. The effectiveness of vasodilating agents in ‘arterializing’ earlobe capillary blood samples seems not to have been formally assessed.
References+ View more
- Eisen L, Miami T, Berger J et al. Gender disparity in failure rate for arterial catheter attempts. J Intensive Care 2007; 22: 166–72.
- Wallach SG. Cannulation injury of the radial artery: diagnosis and treatment algorithm. Am J Crit Care 2004; 13: 315–19.
- Okeson G, Wullbrecht P. The safety of brachial artery puncture for arterial blood sampling. Chest 1998; 114: 748–51.
- Giner J, Casan P, Belda J et al. Pain during arterial puncture. Chest 1996; 110: 1443–45.
- Dar K, Williams T, Aitken R et al. Arterial versus capillary sampling for analysing blood gas pressures. BMJ 1995; 310: 24–5
- Paes B, Janes M, Vegh P et al. A comparative study of heel stick devices for infant blood collection. Am J Dis Child 1993; 147: 346–48.
- Hughes J. Blood gas estimations from arterialized capillary blood versus arterial puncture: are they different? Eur Respir J 1996; 9: 184–85.
- Toftegaard M, Rees S, Andreeason S. Correlation between acid-base paramenters measured in arterial blood and venous blood sampled peripherally, from the vena cava and from the pulmonary artery. Eur J Emerg Med 2008; 15: 86–91.
- Burnett RW, Covington AK, Fogh-Anderson N et al (IFCC) Approved IFCC recommendations on whole blood sampling, transport and storage for simultaneous determination of pH blood gases and electrolytes. Eur J Chem Clin Biochem 1995; 33: 247–53.
- Sauty A, Uldry C, Debatez L-F. Differences in pO2 and pCO2 between arterial and arterialised earlobe samples. Eur Respir J 1996; 9: 186–89.
- Dong SH, Liu HM, Song GW et al. Arterialised capillary blood gases from acid-base studies in normal individuals from 29 days to 24 years of age. Am J Dis Child 1985; 139: 1019–22.
- Cousineau J, Anctil S, Carcellar A et al. Neonatal capillary blood gas reference values. Clin Biochem 2005; 38: 905–07.
- MacRae DJ, Palavradi D. Comparison between arterial, capillary and venous acid-base measurements in the newborn infant. J Obstet Gynae of Br Commonwealth 1966; 73: 761–65.
- Desai S, Holloway R, Thambiran A et al. A comparison between arterial and arterialised capillary blood in infants. S Afr Med J 1967; 41: 13–15.
- Hunt C. Capillary blood sampling in the infant: usefulness and limitations of two methods of sampling compared with arterial blood. Pediatrics 1973; 51: 501–06.
- McLain B, Evans J, Dear P. Comparison of capillary and arterial blood gas measurements in neonates. Arch Dis Child 1988; 63: 743–47.
- Harrison A, Lynch J, Dean J. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care patients. Crit Care Med 1997; 25: 1904–08.
- Johnson K, Cress G, Connolly N et al. Neonatal Laboratory blood sampling: comparison of results from arterial catheters with those from an automated capillary device. Neonatal Network 2000; 19: 27–34.
- Escalante-Kanashiro R, Tantalean-Da-Fieno J. Capillary blood gases in a pediatric intensive care unit. Crit Care Med 2000; 28: 224–26.
- Yang K, SU B-H, Tsai F-J, Peng C-T. The comparison between capillary blood sampling and arterial blood sampling in an NICU. Acta Paediatr Taiwan 2002; 43: 124–26.
- Yildizas D, Yapicioglu H, Yilmaz H et al. Correlation of simultaneously obtained capillary, venous and arterial blood gases of patients in a paediatric intensive care unit. Arch Dis Child 2004; 89: 176–80.
- Pitkin A, Roberts C, Wedzicha J. Arterialised earlobe blood gas analysis: an underused technique. Thorax 1994; 49: 364–66.
- Dar K, Williams T, Aitlen R. Arterial versus capillary sampling for analysing blood gas pressures. BMJ 1995; 310: 24–5.
- Dall’Ava-Santucci J, Dessanges J, Dinh Xaun A et al. Is arterialised earlobe blood pO2 an acceptable substitute for arterial blood pO2? Eur Respir J 1996; 9: 1329–30.
- Fajac I, Texereau V, Rivoal J-F et al. Blood gas measurement during exercise: a comparative study between arterialized earlobe sampling and direct arterial puncture in adults. Eur Respir J 1998; 11: 712–15.
- Eaton T, Rudkin S, Garrett J. The clinical utility of arterialised earlobe capillary blood in the assessment of patients for long term oxygen therapy. Respir Med 2001; 95: 655–60.
- Zavorsky G, Cao J, Mayo N. Arterial versus capillary blood gases: a meta- analysis. Respir Physiol & Neurobiology 2007; 155: 268–79.
- Janes M, Pinelli J, Landry S et al. Comparison of capillary blood sampling using an automated incision device with and without warming the heel. J Perinatology 2002; 22: 154–58.
- Barker DP, Willets B, Cappendijik V et al. Capillary blood sampling: should the heels be warmed? Arch Dis Child (Fetal & Neonatal) 1996; 74: F139–140.
- Gandy G, Grann L, Cunningham N et al. The validity of pH and pCO2 measurements in capillary samples in sick and healthy infants. Pediatrics 1964; 34: 192–97.
References
- Eisen L, Miami T, Berger J et al. Gender disparity in failure rate for arterial catheter attempts. J Intensive Care 2007; 22: 166–72.
- Wallach SG. Cannulation injury of the radial artery: diagnosis and treatment algorithm. Am J Crit Care 2004; 13: 315–19.
- Okeson G, Wullbrecht P. The safety of brachial artery puncture for arterial blood sampling. Chest 1998; 114: 748–51.
- Giner J, Casan P, Belda J et al. Pain during arterial puncture. Chest 1996; 110: 1443–45.
- Dar K, Williams T, Aitken R et al. Arterial versus capillary sampling for analysing blood gas pressures. BMJ 1995; 310: 24–5
- Paes B, Janes M, Vegh P et al. A comparative study of heel stick devices for infant blood collection. Am J Dis Child 1993; 147: 346–48.
- Hughes J. Blood gas estimations from arterialized capillary blood versus arterial puncture: are they different? Eur Respir J 1996; 9: 184–85.
- Toftegaard M, Rees S, Andreeason S. Correlation between acid-base paramenters measured in arterial blood and venous blood sampled peripherally, from the vena cava and from the pulmonary artery. Eur J Emerg Med 2008; 15: 86–91.
- Burnett RW, Covington AK, Fogh-Anderson N et al (IFCC) Approved IFCC recommendations on whole blood sampling, transport and storage for simultaneous determination of pH blood gases and electrolytes. Eur J Chem Clin Biochem 1995; 33: 247–53.
- Sauty A, Uldry C, Debatez L-F. Differences in pO2 and pCO2 between arterial and arterialised earlobe samples. Eur Respir J 1996; 9: 186–89.
- Dong SH, Liu HM, Song GW et al. Arterialised capillary blood gases from acid-base studies in normal individuals from 29 days to 24 years of age. Am J Dis Child 1985; 139: 1019–22.
- Cousineau J, Anctil S, Carcellar A et al. Neonatal capillary blood gas reference values. Clin Biochem 2005; 38: 905–07.
- MacRae DJ, Palavradi D. Comparison between arterial, capillary and venous acid-base measurements in the newborn infant. J Obstet Gynae of Br Commonwealth 1966; 73: 761–65.
- Desai S, Holloway R, Thambiran A et al. A comparison between arterial and arterialised capillary blood in infants. S Afr Med J 1967; 41: 13–15.
- Hunt C. Capillary blood sampling in the infant: usefulness and limitations of two methods of sampling compared with arterial blood. Pediatrics 1973; 51: 501–06.
- McLain B, Evans J, Dear P. Comparison of capillary and arterial blood gas measurements in neonates. Arch Dis Child 1988; 63: 743–47.
- Harrison A, Lynch J, Dean J. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care patients. Crit Care Med 1997; 25: 1904–08.
- Johnson K, Cress G, Connolly N et al. Neonatal Laboratory blood sampling: comparison of results from arterial catheters with those from an automated capillary device. Neonatal Network 2000; 19: 27–34.
- Escalante-Kanashiro R, Tantalean-Da-Fieno J. Capillary blood gases in a pediatric intensive care unit. Crit Care Med 2000; 28: 224–26.
- Yang K, SU B-H, Tsai F-J, Peng C-T. The comparison between capillary blood sampling and arterial blood sampling in an NICU. Acta Paediatr Taiwan 2002; 43: 124–26.
- Yildizas D, Yapicioglu H, Yilmaz H et al. Correlation of simultaneously obtained capillary, venous and arterial blood gases of patients in a paediatric intensive care unit. Arch Dis Child 2004; 89: 176–80.
- Pitkin A, Roberts C, Wedzicha J. Arterialised earlobe blood gas analysis: an underused technique. Thorax 1994; 49: 364–66.
- Dar K, Williams T, Aitlen R. Arterial versus capillary sampling for analysing blood gas pressures. BMJ 1995; 310: 24–5.
- Dall’Ava-Santucci J, Dessanges J, Dinh Xaun A et al. Is arterialised earlobe blood pO2 an acceptable substitute for arterial blood pO2? Eur Respir J 1996; 9: 1329–30.
- Fajac I, Texereau V, Rivoal J-F et al. Blood gas measurement during exercise: a comparative study between arterialized earlobe sampling and direct arterial puncture in adults. Eur Respir J 1998; 11: 712–15.
- Eaton T, Rudkin S, Garrett J. The clinical utility of arterialised earlobe capillary blood in the assessment of patients for long term oxygen therapy. Respir Med 2001; 95: 655–60.
- Zavorsky G, Cao J, Mayo N. Arterial versus capillary blood gases: a meta- analysis. Respir Physiol & Neurobiology 2007; 155: 268–79.
- Janes M, Pinelli J, Landry S et al. Comparison of capillary blood sampling using an automated incision device with and without warming the heel. J Perinatology 2002; 22: 154–58.
- Barker DP, Willets B, Cappendijik V et al. Capillary blood sampling: should the heels be warmed? Arch Dis Child (Fetal & Neonatal) 1996; 74: F139–140.
- Gandy G, Grann L, Cunningham N et al. The validity of pH and pCO2 measurements in capillary samples in sick and healthy infants. Pediatrics 1964; 34: 192–97.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Minimizing pre-analytical errors in capillary blood sample collection and handling
Presented by Martha Lyon, PhD, Clinical Biochemist, Royal University Hospital, Saskatoon, Saskatchewan, Canada Watch the webinarScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars









