Printed from acutecaretesting.org
December 1998
Clinical application of transcutaneous oxygen tens
INTRODUCTION
Transcutaneous oxygen measurement (ptcO2, TCOM, tcpO2) has become a popular non-invasive tool for wound assessment and selection of patients for hyperbaric oxygen (HBO) treatment (Fig. 1).
Transcutaneous pO2 measurement was originally used in neonatology [1], and is now commonly used in pediatrics ICU [2], plastic surgery [3], vascular surgery [4], anesthesiology [5], orthopedics [6], and hyperbaric medicine [7].
In 1994, Matos and Nunez [8] reviewed a series of tissue oxygenation studies and concluded that tcpO2 was clinically useful in determining healing potential, selecting amputation level, evaluating revascularization procedures, and assessing severity and progression of peripheral vascular disease.
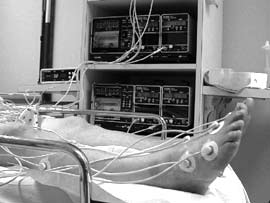 |
FIG. 1a |
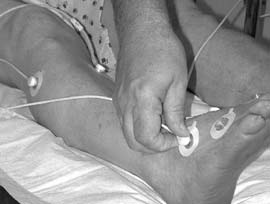 |
FIG. 1b |
FIG 1a and 1b: Application of tc electrodes for assessment of a patient for hyperbaric oxygen treatment (photos by D. A. Davoli).
In order to aid oxygen migration to the skin surface where it can be analyzed, the non-invasive sensor must cause physiological changes in the underlying tissue.
Heating the sensor to 42-45 oC transfers heat to the skin surface that dilates capillaries, opens skin pores, decreases oxygen solubility, and shifts the oxyhemoglobin curve to the right for a more ready release of oxygen [9].
In the absence of heat, diffusion of oxygen from tissue to skin surface contributes less than 3.5 mmHg to the pO2 at that location [10]. At present, transcutaneous sensors will not adhere to a moist surface, so tcpO2 values are collected near the wound.
Assessment of the data’s significance to the wound requires careful interpretation. A control sensor is placed on the skin at the second intercostal space of the chest.
TISSUE OXYGEN ASSESSMENT WITH TRANSCUTANEOUS pO2
|
Ambient pressure (atm abs)/ |
1.0 air |
1.0 O2 |
2.0 O2 |
2.4 O2 |
|
Representative tissue oxygen tension values (mmHg) |
||||
|
Chest [11] |
67±12 |
450±54 |
— |
1312±112 |
|
Calf, Male [11] |
49±14 |
281±78 |
— |
1027±164 |
|
Calf, Female [11] |
59±12 |
367±59 |
— |
1174±127 |
|
Mid foot [11] |
63±13 |
280±82 |
— |
919±214 |
|
Limb [12] |
49 |
325 |
696 |
— |
TABLE I: tcpO2 values in healthy subjects at progressively increased inspired pO2.
Normal tissue oxygen tension
Table I contains “normal” tcpO2 values of Dooley et al [11], obtained from 72 subjects (53 males, 19 females) at the chest, calf, and mid foot. It reveals a significant gender difference at the calf. Data obtained at 2 atm abs were reported by Hart et al [12].
tcpO2 as a predictor of successful amputation site without HBO
A number of patient studies without HBO confirmed that normal healing of an amputation site requires a tcpO2 value of at least 40 mmHg [13], or regional perfusion index (RPI = limb/chest tcpO2) of about 0.6 [14].
White & Klein [13] reviewed 8 patient studies of transcutaneous pO2 values for 260 amputees and concluded that a tcpO2 value of 40 mmHg or greater was required for a successful outcome of amputation. Hauser [14] assessed 159 wounds (93 local débridements and 66 amputations) in 113 high-risk diabetic patients with peripheral vascular disease. tcpO2 values were obtained at four sites on the limbs and compared to chest controls to obtain the regional perfusion index (RPI = wound tcpO2/chest tcpO2).
Hauser reported excellent outcome when RPI > 0.6, and poor outcome when RPI < 0.4 [14].
tcpO2as a predictor of wound healing with HBO
Monitors from several manufacturers have been used effectively for transcutaneous oxygen studies [15]. Currently, the TINA, TCM3 and TCM30 (Radiometer Medical A/S, Copenhagen) are the only monitors with sensors that have been tested and shown to be compatible with pure-oxygen chambers [15].
Fire safety considerations require the monitor to remain outside a pure-oxygen chamber. There is controversy as to the predictive value of tcpO2 taken at atmospheric pressure or under hyperbaric conditions.
tcpO2 under atmospheric conditions
Pecoraro et al [16] found tcpO2 values to be useful as predictors of healing in diabetic patients, and in selecting patients for adjunctive HBO to correct underlying tissue hypoxia, either alone or in combination with revascularization.
Brakora and Sheffield [17] concluded that diabetic patients whose periwound tcpO2 values are above 40 mmHg (non-diabetic patients above 30 mmHg) should have sufficient tissue oxygenation to heal.
Conversely, diabetic patients whose periwound tcpO2 values are below 40 mmHg (non-diabetic patients below 30 mmHg) were considered to have tissue hypoxia appropriate for HBO, provided there was a significant rise in tcpO2 during oxygen challenge. Gorman [18] conducted a review of diabetic foot wounds and concluded that patients who have relative tcpO2 values greater than 85 % (% of chest control) and ankle pressure greater than 90 mmHg will heal without oxygen.
Conversely, those with relative tcpO2 values less than 20 % and ankle pressure less than 75 mmHg are unlikely to heal. Using Gorman’s criteria, candidates for HBO would be those patients whose relative tcpO2 values were in the range 20 to 85 % with an ankle pressure between 75-90 mmHg. S
heffield and Workman [19] reported improved baseline tcpO2 at the wound site taken at 7-day intervals while the patients received HBO therapy.
Marx et al [20] achieved an RPI of 0.8 in irradiated tissue from the head and neck within four weeks of starting HBO therapy, and established a protocol of 20 preoperative HBO treatments before reconstruction of the mandible.
Sheffield [21] retrospectively found that diabetic patients with forefoot wounds (n = 84) had an 8-fold increase in likelihood of successful outcome with HBO, when baseline transmetatarsal tcpO2 values were greater than 30 mmHg as compared to tcpO2 values less than 30 mmHg (p < 0.05).
tcpO2 under hyperbaric conditions
In 1983, Sheffield and Workman [22,23] used a Radiometer TCM1 monitor to record the first known tcpO2 data under hyperbaric conditions (100 % O2 at 2.4 atm abs, 238 kPa), reporting values above 1000 mmHg.
Recently a number of investigators have suggested that the best predictor of wound healing success might be tcpO2 conducted under hyperbaric conditions, but their suggested absolute values varied widely.
Myers and Emhoff [24] reported that diabetic patients (n = 11) with below-the-knee tcpO2 values less than 20 mmHg, would heal if 900 to 1,100 mmHg could be achieved on the initial HBO exposure. Wattel et al [25] concluded that tcpO2 values above 450 mmHg during HBO were predictive of healing in diabetic patients with plantar ulcers.
Campagnoli et al [26] reported that diabetic patients (n = 24) healed if tcpO2 values were above 400 mmHg during HBO, and observed that the faster the rise, the greater the likelihood of an efficient support microcirculation, which would improve the chance of a favorable outcome.
Strauss et al [27] measured tcpO2 in 87 patients with problem foot wounds and reported that 98 % healed when tcpO2 measured over 200 mmHg during HBO (100 % O2 for 90 min at 2 atm abs, 202 kPa).
tcpO2 assessment method
Several methods have been used to assess tcpO2 to predict healing potential: 1) a single tcpO2 value taken adjacent to the wound; 2) a map of multiple sites around the wound; 3) a map of several sites on the affected limb; and 4) a comparison of periwound or amputation-site values expressed as a percentage of chest control values.
The preferred method seems to be a map of at least three sites. But regardless of the assessment method, it is important to be consistent. There should be a standard approach to positioning the sensor that accounts for the normal circulation to the limb.
This will provide more consistent data and allow data among a group of patients to be compared. Our tcpO2 assessment procedure and a typical tcpO2 map of standardized sites on the legs are shown in Table II and Fig. 2, respectively.
Table III gives an example of the results obtained using this tcpO2 assessment procedure.
|
TABLE II: tcpO2 assessment procedure for mapping the skin surface. |
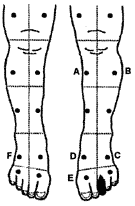 |
FIG 2: Typical tcpO2 mapping of the legs with six electrodes plus chest control. |
Interpretation of data
Some investigators [17,25,27] predict success by interpreting the actual tcpO2 values. Others [14,18,20] calculate a regional perfusion index (RPI = limb tcpO2/chest tcpO2). There is no consensus on the best method for collecting or interpreting the data.
However, it is clear that interpretation demands careful assessment of the tissue on which the sensor is placed. tcpO2 values can be elevated if the sensor is positioned over an artery, or if there is a leak under the fixation ring.
On the other hand they, can be lowered if the sensor is positioned over bone, or if the patient uses tobacco products. tcpO2 values in smokers were 10 % below that of non-smokers [22], and remained significantly reduced for about an hour after smoking [28].
But there is evidence of improved tissue oxygenation after a few days of smoking cessation [29]. tcpO2 values may also be lowered by several pathological conditions: edema, active infection or inflammation, thick or sclerotic skin, occluded vessels, severed vessels (flap), or irradiated tissue.
The data interpreter must consider all these factors. The physician interpreter of the tcpO2 data in Fig. 2 considered the hypoxic tissue near the wound that responded to the oxygen challenge, and recommended HBO to prevent further necrosis and to help control infection [21].
|
REGION |
Con- |
Elec- |
Elec- |
Elec- |
Elec- |
Elec- |
Elec- |
|
|
tcpO2 (mmHg) |
||||||||
|
Supine |
20 min. Air |
57 |
44 |
50 |
2 |
45 |
9 |
58 |
|
45 degree Elev. |
5 min. Air |
54 |
25 |
39 |
1 |
24 |
2 |
39 |
|
Supine |
5 min. Air |
50 |
40 |
41 |
2 |
43 |
7 |
49 |
|
Supine |
10 min. O2 |
290 |
79 |
88 |
4 |
178 |
40 |
67 |
|
Supine |
5 min. Air |
96 |
48 |
47 |
3 |
47 |
10 |
62 |
TABLE III: Example of the results obtained after assessment of tcpO2 using the electrode mapping depicted in Fig. 2.
SUMMARY
Sufficient clinical experience is being published to justify transcutaneous pO2 measurement, but the procedures require rigorous protocol and the interpreter must consider the limits of the technology.
Best results are obtained from a dedicated, qualified technician who measures at standardized sites on the limb. The data suggests that the best predictor of success with HBO-treated cases might be tcpO2 data collected under hyperbaric conditions.
Author
Paul J. Sheffield
International ATMO, Inc.
4499 Medical Drive, SL2
San Antonio, Texas 78229
USA
References+ View more
- Huch R, Huch A. Crit Care Med 1981; 9 10: 694-97.
- Ravindranath T. Indian J Pediatr, 1990; 57: 169-73.
- Serafin D, Lesense CV, Mullen RY, et al. Experimental and clinical correlations. J Microsurg 1981; 2 3: 165-78.
- Rooke TW. Int. Angiol 1992; 11, 1: 36-40.
- Dennhardt R, Ricke MF, Huch A, et al. Eur J Intens Care Med 1976; 2: 29-33.
- Wyss CR, Harrington RM, Burgess EM et al. J Bone and Joint Surg 1988; 7A(2): 203-07.
- Simanonok J. Triage 1996; VIII, 4: 1,7
- Matos LA, Nunez AA. In: Kindwall EP, ed). Hyperbaric medicine practice. Flagstaff AZ: Best Publishing, 1994: 589-612.
- Lubbers DW. Crit Care Med 1981; 9: 721-33.
- Evans NTS, Naylor PFD. Resp Physiol 1967; 3: 21-37.
- Dooley J, King G, Slade B. Undersea Hyperb Med 1997; 24, 4: 235-44.
- Hart GB, Meyer GW, Strauss MB et al. J Hyperb Med 1990; 5(4): 223-29.
- White RA and Klein SR. In: Moore WS and Malone JM, eds. Lower extremity amputation. Philadelphia, PA: WB Saunders, 1989; 44-49.
- Hauser CJ. Arch Surg 1987; 22: 1128-30.
- Marotte S, Larson-Lohr V, Weaver LK. Undersea Hyperb Med 1993; 20 Suppl: 28
- Pecoraro RE, Ahroni JH, Boydo EJ, Stensel VL. Diabetes 1991; 40: 1305-12.
- Brakora MJ, Sheffield PJ. In: LA Lavery, KJ Dennis, eds. Clinics in podiatric medicine and surgery: the diabetic foot. Philadelphia, PA: WB Saunders, 1995; 12, 1: 105-17.
- Gorman DF. In: Frykberg RG, ed. The high risk foot in diabetes mellitus. New York, NY: Churchill Livingstone, 1991: 441-47.
- Sheffield PJ, Workman WT. Hyperb Oxyg Rev. 1985; 6, 1: 47-62.
- Marx, RE, Johnson RD, Kline. J Am Dent Assoc III 1985; 49-54.
- Sheffield PJ. Undersea Hyperb Med 1998; 25, 3: 179-88.
- Workman WT, Sheffield PJ. In: Huch R, Huch A, eds. Continuous transcutaneous blood gas monitoring. New York: Marcel Dekker, 1983: 649-56.
- Sheffield PJ, Workman, WT. In: Huch R, Huch A, eds. Continuous transcutaneous blood gas monitoring. New York: Marcel Dekker, 1983: 667-72.
- Myers RAM, Emhoff TA. In: Program and abstracts. Eight International Congress on Hyperbaric Medicine, Bethesda, MD: Undersea Medical Society, 1984: 185-86.
- Wattel FE, Mathieu MD, Fossati P, Neviere RR, Coget JM. Search for healing predictive factors. J Hyperb Med 1991; 6, 4: 263-68.
- Campagnoli P, Oriani G, Sala G. et al. J Hyperb Med 1992; 7, 4: 223-27.
- Strauss MJ, Breedlove JW, Hart GB. Undersea Hyperb Med 1997; 24, Suppl: 15.
- Jensen JA, WH Goodson, HW Hopf, & TK Hunt. Arch Surg 1991 126: 1131-34.
- Strauss AG, Hart GB, Strauss MB. Undersea Hyperb Med 1997; 24, suppl: 36.
References
- Huch R, Huch A. Crit Care Med 1981; 9 10: 694-97.
- Ravindranath T. Indian J Pediatr, 1990; 57: 169-73.
- Serafin D, Lesense CV, Mullen RY, et al. Experimental and clinical correlations. J Microsurg 1981; 2 3: 165-78.
- Rooke TW. Int. Angiol 1992; 11, 1: 36-40.
- Dennhardt R, Ricke MF, Huch A, et al. Eur J Intens Care Med 1976; 2: 29-33.
- Wyss CR, Harrington RM, Burgess EM et al. J Bone and Joint Surg 1988; 7A(2): 203-07.
- Simanonok J. Triage 1996; VIII, 4: 1,7
- Matos LA, Nunez AA. In: Kindwall EP, ed). Hyperbaric medicine practice. Flagstaff AZ: Best Publishing, 1994: 589-612.
- Lubbers DW. Crit Care Med 1981; 9: 721-33.
- Evans NTS, Naylor PFD. Resp Physiol 1967; 3: 21-37.
- Dooley J, King G, Slade B. Undersea Hyperb Med 1997; 24, 4: 235-44.
- Hart GB, Meyer GW, Strauss MB et al. J Hyperb Med 1990; 5(4): 223-29.
- White RA and Klein SR. In: Moore WS and Malone JM, eds. Lower extremity amputation. Philadelphia, PA: WB Saunders, 1989; 44-49.
- Hauser CJ. Arch Surg 1987; 22: 1128-30.
- Marotte S, Larson-Lohr V, Weaver LK. Undersea Hyperb Med 1993; 20 Suppl: 28
- Pecoraro RE, Ahroni JH, Boydo EJ, Stensel VL. Diabetes 1991; 40: 1305-12.
- Brakora MJ, Sheffield PJ. In: LA Lavery, KJ Dennis, eds. Clinics in podiatric medicine and surgery: the diabetic foot. Philadelphia, PA: WB Saunders, 1995; 12, 1: 105-17.
- Gorman DF. In: Frykberg RG, ed. The high risk foot in diabetes mellitus. New York, NY: Churchill Livingstone, 1991: 441-47.
- Sheffield PJ, Workman WT. Hyperb Oxyg Rev. 1985; 6, 1: 47-62.
- Marx, RE, Johnson RD, Kline. J Am Dent Assoc III 1985; 49-54.
- Sheffield PJ. Undersea Hyperb Med 1998; 25, 3: 179-88.
- Workman WT, Sheffield PJ. In: Huch R, Huch A, eds. Continuous transcutaneous blood gas monitoring. New York: Marcel Dekker, 1983: 649-56.
- Sheffield PJ, Workman, WT. In: Huch R, Huch A, eds. Continuous transcutaneous blood gas monitoring. New York: Marcel Dekker, 1983: 667-72.
- Myers RAM, Emhoff TA. In: Program and abstracts. Eight International Congress on Hyperbaric Medicine, Bethesda, MD: Undersea Medical Society, 1984: 185-86.
- Wattel FE, Mathieu MD, Fossati P, Neviere RR, Coget JM. Search for healing predictive factors. J Hyperb Med 1991; 6, 4: 263-68.
- Campagnoli P, Oriani G, Sala G. et al. J Hyperb Med 1992; 7, 4: 223-27.
- Strauss MJ, Breedlove JW, Hart GB. Undersea Hyperb Med 1997; 24, Suppl: 15.
- Jensen JA, WH Goodson, HW Hopf, & TK Hunt. Arch Surg 1991 126: 1131-34.
- Strauss AG, Hart GB, Strauss MB. Undersea Hyperb Med 1997; 24, suppl: 36.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









