Printed from acutecaretesting.org
July 2021
COVID-19 pandemic — the tide is turning
The emerge of a pandemic
On December 30th, 2019 Dr. Li Wenliang reported in a web chat with colleagues a cluster of seven patients with severe pneumonia in Wuhan, China. He also noted that all seven patients had attended the Huanan seafood market, likely one of the first superspreading events of coronavirus [1].
His report was picked-up by WHO and other health care organizations. Wheels were set in motion. Within two weeks, the RNA sequence of SARS-CoV-2 was determined and shared with the international community. The disease became its official name —COVID-19 (coronavirus disease 2019), and the first guidance for identification and prevention was distributed by the WHO.
On January 11th, 2020, China reported the first death from COVID-19, as the virus began its march across the world. On March 11th, COVID-19 was declared a pandemic by WHO, with around 60,000 confirmed cases worldwide, and over 2000 deaths. Little did we know then, that mankind was about to face one of the most devastating infectious diseases encountered in modern times.
Today, towards the end of spring 2021, the tide is turning. Where vaccination programs have started to kick-in, life is returning to normal. Yet, in many countries in Africa, Asia, and South America, full-scale vaccination programs are still months away and the fight against the pandemic far from over. Moreover, the continuous emerge of new variants might pose to be a recurrent threat for years to come.
At this writing, about 170 million people have been infected with the coronavirus, more than 3.5 million individuals have died, and the counting continues.[2]
Current understanding of COVID-19
The disease
Coronavirus particles are airborne in small droplets (aerosols) which are spread through talking, sneezing, singing, and so on. Social distancing and wearing protective mouth/nose caps and coverings are therefore the most effective measures to reduce the risk of transmission [3]. Primary infection sites are the mucosa in the nose, mouth, and respiratory tract, as well as the eyes. Although other ways have been reported, transmission of COVID-19 is typically achieved by exposure to contaminated aerosols, either direct through inadequate social distancing, or indirect by inhaling contaminated air in an unventilated room where an infected person is or was present [1]. Also touching a contaminated surface and then touching mouth or eyes with hand has been reported as a way of transmission. Regular room ventilation, handwashing, and cleaning of potentially contaminated surfaces are therefore also part of the hygiene measures to contain the spread of coronavirus [3].
After an incubation time of 1-12 days, some early common signs of COVID-19 are a dry cough, fever, feeling tired, and a loss of taste/smell. More early symptoms are listed below [4]:
| Symptom | Prevalence (%) |
| Dry cough | 60.4 |
| Shortness of breath og breating difficulties | 41.1 |
| Fever | 55.5 |
| Mucle pain | 44.6 |
| Headache | 42.6 |
| Sore throat | 31.2 |
| Smell and taste disturbance | 64.4 |
| Fatigue | 68.3 |
Most COVID-19 patients will experience a mild course of the infection and do not need any medical treatment. As a rule of thumbs, 20% of all COVID-19 patients will require medical attention and/or hospitalization, of which 20% eventually require ICU treatment, often for mechanical ventilation. Depending on the level of co-morbidities, age, gender, and other variables, mortality in this latter group was found up to 72% [5].
The virus
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus type 2) is the virus causing COVID-19 and the seventh coronavirus known to infect humans; SARS-CoV, MERS-CoV and SARS-CoV-2 can cause severe disease, whereas HKU1, NL63, OC43 and 229E are associated with mild symptoms. The origin of SARS-CoV-2 is suspected to be found in wildlife [6].
An overview of the virus life cycle and immune response is depicted below. (Figure reproduced with permission of Funk et al. [7]
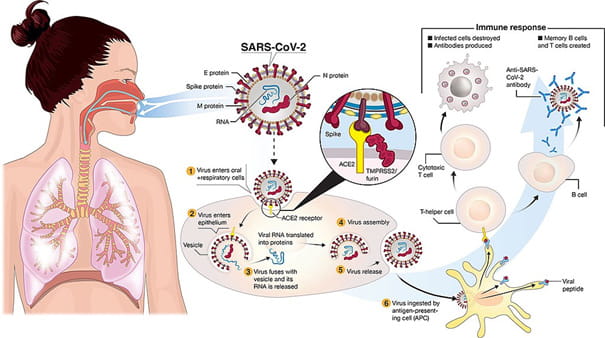
In short, SARS-CoV-2 is transmitted to oral and respiratory mucosal cells. The virus, possessing a single-stranded RNA genome wrapped in nucleocapsid (N) protein and three major surface proteins: membrane (M), envelope (E) and Spike, replicates and passes to the lower airways potentially leading to severe pneumonia. The gateway to host cell entry (magnified view) is via Spike-Angiotensin converting enzyme 2 (ACE2) interaction with cleavage of Spike in the prefusion state by proteases TMPRSS-2/furin. A simplified depiction of the life cycle of the virus is shown along with a potential immune response in which especially T-cells seem to play a crucial role [8].
As there is abundant surface expression of ACE2 on lung alveolar epithelial cells and enterocytes of the small intestine, these sites provide suited receptors and points-of-entry for SARS-CoV2. Furthermore, ACE2 is found present in arterial and venous endothelial cells and arterial smooth muscle cells in many organs, including kidney, liver, and heart [9]. This systemic distribution of the ACE2 is a possible explanation why SARS-CoV-2, compared to other types of SARS, is relatively often associated with multi-organ failure [10].
Variants of concern
SARS-CoV-2 is a single-stranded RNA-based (ribonucleic acid) virus and therefore vulnerable to mutations. More than 6000 mutations of the trimeric S(pike) protein alone have been reported, mutations that will affect transmissibility of the virus. Most of the mutations result in loss-of-function or decreased-function mutations rendering variations which do not play a part in the further spread of the virus. However, occasionally such mutations cause the new variant to be more contagious or cause more severe disease (gain-of-function) than the wild-type [11]. Such superior variants will replace the wild-type during a pandemic, as has happened with the original Wuhan strain.
Currently, all coronavirus variants are a member of the G-clad strain, e.g., contain the D614G mutation, while 4 variants are classified “of concern”, meaning these variants are associated with at least one of the following gain-of-function characteristics: that it transmits more easily; it causes more severe illness; it significantly reduces neutralization by antibodies; or reduces the effectiveness of treatment, vaccines, or diagnosis.
Variants of concern [12]
| Lineage (WHO) | First documented | Attributes |
| B.1.1.7 (Alpha) | United Kingdom Sep. 2020 |
|
| B.1.351 (Beta) | South Africa May 2020 |
|
| P.1 (Gamma) | Brazil Nov. 2020 |
|
| B.1.617.2 (Delta) | India Oct. 2020 |
|
*EUA: Emergency Use Authorization for US market (FDA)
The “delta” variant uncovered in India/UK (B.1.617.2) has been upgraded recently by WHO from a variant of interest to a variant of concern. Currently, this variant is spreading rapidly in countries like UK, Russia and Portugal. Because of its higher virulence compared to the other variants, this “delta” variant is expected to develop into the most prevalent strain causing COVID-19. Also other new mutations based on a mix of delta and gamma have been reported.
Variants of interest
Other variants are currently graded as variants of interest and are monitored carefully, even when these variants are currently not very prevalent. Such variants are the Epsilon and Iota in USA, Zeta in Brazil, Theta in the Philippines, Kappa in India, Lambda in Peru, and Eta in several countries.
Variants of high consequence
A variant will be classified as variant of high consequence when clear evidence indicates that current prevention measures or medical countermeasures have significantly reduced effectiveness for this variant relative to previously circulating variants. So far, no SARS-CoV-2 variants of High Consequence have emerged.
How to detect SARS-CoV-2 infection
There are basically two ways for detecting infection with coronavirus.
- Detecting SARS-CoV-2 antigen (Ag); the principle of immunoassays shared by practically all rapid screening- and self-tests. Briefly, a sample (extract from nose swap) is brought on a strip with immobilized coronavirus antibodies (Ab). When Ab-Ag complexes are formed, indicated by a color change, the test is positive for the presence of coronavirus. Although these tests are suited for screening and render results often within a few minutes, false negatives as well as false positives have been reported [13, 14]. Confirmation of a positive immunoassay result by means of a RT-PCR (Reverse Transcription Polymerase Chain Reaction) assay is therefore recommended.
- Detecting SARS-CoV-2 RNA; in short a sample (extract from a nose or throat swap) is submitted to a RT-PCR cycler to amplify the viral RNA exponentially If SARS-CoV-2 RNA is present in the sample, one of the chemicals in the process produces a fluorescent light. Diagnostic accuracy of PCR-based assays is superior to the immunoassays [14].
Serology tests quantify circulating antibodies to SARS-CoV2 (IgG, IgM), thereby indicating an immune response caused by COVID-19 or recent vaccination. As it typically takes about a week for an infected individual to develop enough antibodies to be detected, antibody testing is unsuited to diagnose an active infection, but might serve to assess current immune status in a patient, as well as in the general population [14]. Also, the test can determine the Ab-titer in convalescent plasma [14].
Management of COVID-19
Once the diagnosis of COVID-19 is ascertained, clinical management of the patients depends on severity of the disease [15].
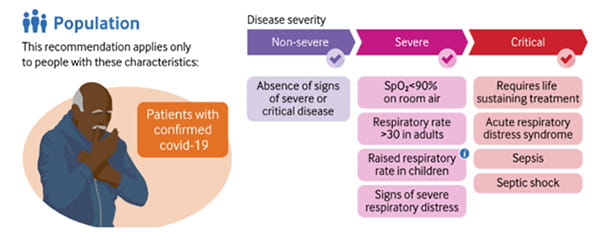
i) ≥ 60 in children under 2 months. ≥ 50 in children between 2-11 months. ≥ 40 in children between 1-5 years.
Reproduced from the BMJ Rapid Recommendations (copyright managed by MAGIC).
Non-severe COVID-19
In many patients, especially of a younger age, COVID-19 expresses itself with none or mild symptoms (e.g. dry cough, headache, fever, nausea). In most cases non-severe COVID-19 patients will stay at home (self-isolation), take some anti-symptom medication, and will not require other treatment. After a 2 to 3-week period of watchful waiting, most of these patients are almost recovered and can return to public life.
Unfortunately, around 20% of patients will develop severe COVID-19 requiring home care or hospital admission and treatment. Several risk factors and co-morbidities are known to increase the probability of getting infected, or to develop severe COVID-19. Briefly, middle-aged and elderly have a higher risk for severe COVID-19 and death than younger individuals. More than 80% of COVID-19 deaths occur in people over age 65, and more than 95% of COVID-19 deaths occur in people older than 45 [16].
Other risk factors for severe COVID include high blood pressure, obesity, and smoking.
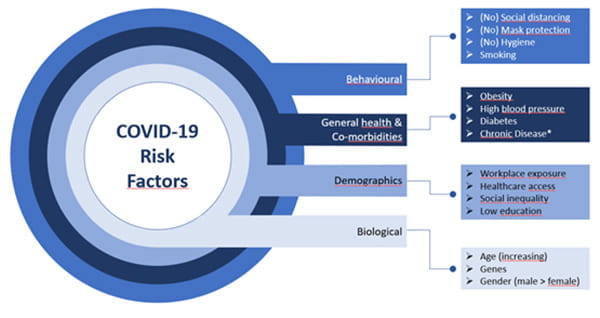
Figure adapted from Roy et al. [17], credit to Molly Patton.
* Patients with chronic disease, such as diabetes, cancer, chronic lung disease (e.g. COPD), cardiovascular disease, immunosuppressed, dementia, mental disorders, and chronic kidney disease, will face an increased risk for developing severe COVID.
Severe COVID-19
In case of severe COVID-19 some degree of lung damage has been inflicted, often associated with respiratory distress and a reduced oxygen saturation level in the blood (< 90% on room air). Severe COVID-19 patients require oxygen support and receive medications to prevent further escalation of the viral infection, attenuate inflammatory processes, and reduce the risk for other complications.
Eventually, a small proportion of severe COVID-19 patients will develop critical COVID-19 and require ventilation and critical care in an ICU.
Critical COVID-19
When larger quantities of lung tissue are irreversibly destroyed, caused primarily by an uncontrolled inflammatory auto-immune response (cytokine storm), then patients will have developed critical COVID-19, often expressed as acute respiratory distress syndrome (ARDS), and require endured intensive care treatment [8].
During a phase of critical COVID-19 vital organs and several biochemical processes are affected. Even when the critical phase has passed after a prolonged stay in the hospital, many patients will return to their home with enduring symptoms of the infection (see Long COVID-19).
An overview of the recommended clinical management of COVID-19 according to current WHO, CDC (USA), and NHS (UK) guidelines is presented below.
| Outpatients | Inpatients | |||
|---|---|---|---|---|
| COVID-19 | Non-severe | Non-severe | Severe (Ward) | Critical (ICU) |
| Symptoms | No / mild | No / mild | Moderate | Severe |
| Risk Factors | No / low | Low / Moderate / High | Moderate / High | |
| Management | Watchfull waiting | Chest X-ray | ||
| Treat symptoms with appropriate drugs | Antibiotics (monitored) | Antibiotics | ||
| Pulse oximetry monitoring | Target SpO2 > 90% (higher for emergency or pregnancy); ventilation if indicated | |||
| Thromboprophylaxis | Thromboprophylaxis | |||
| Anti-SARS-CoV-2 monoclonal antibody therapy | Anti-SARS-CoV-2 monoclonal antibody therapy | |||
| Ensure adequate hemodynamics | ||||
| Dexamethasone plus Remdesivir | ||||
| Lab tests (see below), inflammatory status, liver, kidney, and heart function should be monitored; monitor lung-function through continuous blood gas analysis. Check lactate levels and electrolytes. | ||||
| Without signs of improvement, consider “high-titer” convalescent plasma exchange, ECMO, or investigational therapies | ||||
Laboratory tests
Currently, no well-designed studies have validated the optimal use of laboratory markers in COVID-19 management. Based on published data and experience with management of other infectious diseases, the following recommendations are published by an IFCC task force [18].
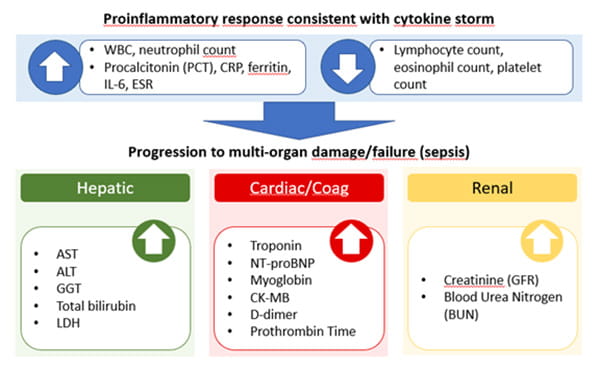
Image adapted from Bohn et al. [17].
Briefly, a regular whole blood count, including WBCdiff, will indicate any major changes in platelet count and in white blood cell counts, e.g. lymphocytes, monocytes, eosinophils, neutrophils, and basophils. The cellular inflammatory markers will help to detect an emerging cytokine storm. CRP and PCT will aid in detection of inflammation and co-infections, respectively. Especially PCT was shown effective in guiding antibiotic treatment in patients with respiratory infections [19].
Liver function biomarkers should be ordered routinely to assess relative hepatic injury, particularly for those receiving antiviral treatment.
The cardiac markers are prognostic and will offer information on heart damage (troponin) and function (NT-proBNP). D-dimer levels have also been shown to be a valid prognostic parameter in COVID-19, while a coagulation imbalance, as observed in COVID-19 patients with thromboembolic complications, can be diagnosed by biomarkers such as D-dimer, Prothrombin, and platelet count.
Finally, acute kidney injury is strongly associated with mortality. It is important to measure renal function regularly over the time of ICU stay (i.e. creatinine (GFR) and BUN).
Long COVID
Long COVID is a condition that can last or appear weeks after first being infected with coronavirus and can happen to anyone even if they had mild symptoms or no symptoms from COVID-19 at all. Recent reporting from German Health Services indicated that 350.000 of around 3.5 million patients with confirmed COVID-19 demonstrated symptoms of Long COVID, or 10%. In patients with a history of severe or critical COVID-19, percentages of post-COVID-19 symptoms are found much higher as reported in several studies across the globe [20, 21, 22]
People with Long COVID report experiencing different combinations of the following symptoms:
- Tiredness or fatigue
- Difficulty thinking or concentrating
- Headache
- Loss of smell or taste
- Dizziness when standing
- Fast-beating or pounding heart (palpitations)
- Chest pain
- Difficulty breathing or shortness of breath
- Cough
- Joint or muscle pain
- Depression or anxiety
- Fever
Moreover, these symptoms worsen after physical or mental activities. Long COVID can be persistent for at least 6 months after discharge [20].
Vaccination, once and for all?
Accordance to the current WHO vaccination dashboard, close to 2 billion doses of vaccine have been administered, with more than 5% of the world population being fully vaccinated. However, the differences in vaccinated populations between countries are large, ranging from percentages of around 60% (Israel) to levels under 1% as found in countries in Africa, South America, and Asia [23].
Currently, three vaccines are FDA approved for the USA market: Pfizer/BioNTech, Moderna, and Johnson & Johnson (Janssen-Cilag International) [16]. For the EU-market, aside the three aforementioned vaccones, also the Astra-Zeneca vaccine has been approved [24]. Other markets often have availability of Chinese, Indian, or Russian vaccines. Except for the J&J vaccine requiring one, all others require two injections, with appropriate window, for adequate vaccination [24].
It is expected that coronavirus (variants) will evolve and stay relevant for humankind such as the influenza virus (‘the flu’). Eventually, regular booster vaccinations against new variants of coronavirus might be needed to keep our defense intact.
Closing remarks
The ongoing vaccination program appears to be the decisive game changer in our fight against COVID-19. Already the first shot seems to attenuate the propagation of the COVID-19 virus, and incidences are decreasing in most parts of the world.
The current best-case scenario for the COVID-19 pandemic will encompass the death of around 3.5 million people, will leave millions more with long-lasting physical problems, and will cause a heavy socio-economic burden on our society.
The tide is indeed turning as the COVID-19 pandemic seems to be receding, leaving us with one important lesson to be learned: as a global community we need to be better prepared in the future as a next tide will surely come.
References+ View more
- WHO convened global study of origins of SARS-CoV-2; China-Part; Joint report. 14 January-10 February 2021.
- WHO dashboard https://covid19.who.int/ ; Visited on May 31st, 2021
- WHO; Coronavirus disease (COVID-19) advice for the public; https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public, Visited on June 7th 2021.
- Spinato G, Fabbris C, Polesel J, et al. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA. 2020;323(20):2089–2090. doi:10.1001/jama.2020.6771
- Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020 Sep;8(9):853-862. doi: 10.1016/S2213-2600(20)30316-7. Epub 2020 Jul 28. PMID: 32735842. (limited free domain)
- Andersen, K.G., Rambaut, A., Lipkin, et al. The proximal origin of SARS-CoV-2. Nat Med 26, 450–452 (2020).
- Funk CD, Laferrière C and Ardakani A (2020) A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic. 2020; Front. Pharmacol. 11:937.
- Melms JC, Biermann J, Huang H, et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021 Apr 29. doi: 10.1038/s41586-021-03569-1. Epub ahead of print. PMID: 33915568.
- Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004 Jun;203(2):631-7. doi: 10.1002/path.1570. PMID: 15141377; PMCID: PMC7167720.
- Fadaka AO, Sibuyi NRS, Adewale OB, et al. Understanding the epidemiology, pathophysiology, diagnosis, and management of SARS-CoV-2. J Int Med Res. 2020 Aug;48(8):300060520949077. doi: 10.1177/0300060520949077.
- Sanjuan R and Domingo-Calap P. Mechanisms of viral mutation. Cell. Mol. Life Sci. (2016) 73:4433–48 DOI 10.1007/s00018-016-2299-6.
- ECDC; https://www.ecdc.europa.eu/en/covid-19/variants-concern; Visited on June 23rd, 2021..
- Dinnes J, Deeks JJ, Adriano A, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8(8):CD013705. doi:10.1002/14651858.CD013705
- Shyu D, Dorroh J, Holtmeyer C, et al. Laboratory Tests for COVID-19: A Review of Peer-Reviewed Publications and Implications for Clinical Use. Mo Med. 2020 May-Jun;117(3):184-195. PMID: 32636542; PMCID: PMC7302033.
- Clinical management of COVID-19 patients: living guidance, 25 January 2021; https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1, Visited on May 27th, 2021.
- CDC; COVID-19 https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html; Visited on May 31st, 2021.
- Roy M. Risk factors impacting your susceptibility to COVID-19 & your ability to control them. https://lifeology.io/covid-19-elements-of-risk/; Visited on May 31st, 2021.
- Bohn MK, Lippi G, Horvath A, et al. Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation of the latest evidence. Clin Chem Lab Med. 2020 Jun 25;58(7):1037-1052. doi: 10.1515/cclm-2020-0722. PMID: 32459192.
- Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10(10):CD007498. PMID: 29025194; PMCID: PMC6485408.
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 Jan 16;397(10270):220-232. doi: 10.1016/S0140-6736(20)32656-8. PMID: 33428867; PMCID: PMC7833295.
- Carfi, A., Bernabei, R., Landi, F. & Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 324, 603–605 (2020).
- Chopra, V., Flanders, S. A. & O’Malley, M. Sixty-day outcomes among patients hospitalized with COVID-19. Ann. Intern. Med. https://doi.org/10.7326/M20-5661 (2020).
- Mathieu, E., Ritchie, H., Ortiz-Ospina, E. et al. A global database of COVID-19 vaccinations. Nat Hum Behav (2021).
- Paul Erlich Institute – Infographics. https://www.pei.de/EN/medicinal-products/vaccines-human/covid-19/covid-19-node.html. Visited on June 23rd, 2021.
References
- WHO convened global study of origins of SARS-CoV-2; China-Part; Joint report. 14 January-10 February 2021.
- WHO dashboard https://covid19.who.int/ ; Visited on May 31st, 2021
- WHO; Coronavirus disease (COVID-19) advice for the public; https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public, Visited on June 7th 2021.
- Spinato G, Fabbris C, Polesel J, et al. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA. 2020;323(20):2089–2090. doi:10.1001/jama.2020.6771
- Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020 Sep;8(9):853-862. doi: 10.1016/S2213-2600(20)30316-7. Epub 2020 Jul 28. PMID: 32735842. (limited free domain)
- Andersen, K.G., Rambaut, A., Lipkin, et al. The proximal origin of SARS-CoV-2. Nat Med 26, 450–452 (2020).
- Funk CD, Laferrière C and Ardakani A (2020) A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic. 2020; Front. Pharmacol. 11:937.
- Melms JC, Biermann J, Huang H, et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021 Apr 29. doi: 10.1038/s41586-021-03569-1. Epub ahead of print. PMID: 33915568.
- Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004 Jun;203(2):631-7. doi: 10.1002/path.1570. PMID: 15141377; PMCID: PMC7167720.
- Fadaka AO, Sibuyi NRS, Adewale OB, et al. Understanding the epidemiology, pathophysiology, diagnosis, and management of SARS-CoV-2. J Int Med Res. 2020 Aug;48(8):300060520949077. doi: 10.1177/0300060520949077.
- Sanjuan R and Domingo-Calap P. Mechanisms of viral mutation. Cell. Mol. Life Sci. (2016) 73:4433–48 DOI 10.1007/s00018-016-2299-6.
- ECDC; https://www.ecdc.europa.eu/en/covid-19/variants-concern; Visited on June 23rd, 2021..
- Dinnes J, Deeks JJ, Adriano A, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8(8):CD013705. doi:10.1002/14651858.CD013705
- Shyu D, Dorroh J, Holtmeyer C, et al. Laboratory Tests for COVID-19: A Review of Peer-Reviewed Publications and Implications for Clinical Use. Mo Med. 2020 May-Jun;117(3):184-195. PMID: 32636542; PMCID: PMC7302033.
- Clinical management of COVID-19 patients: living guidance, 25 January 2021; https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1, Visited on May 27th, 2021.
- CDC; COVID-19 https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html; Visited on May 31st, 2021.
- Roy M. Risk factors impacting your susceptibility to COVID-19 & your ability to control them. https://lifeology.io/covid-19-elements-of-risk/; Visited on May 31st, 2021.
- Bohn MK, Lippi G, Horvath A, et al. Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation of the latest evidence. Clin Chem Lab Med. 2020 Jun 25;58(7):1037-1052. doi: 10.1515/cclm-2020-0722. PMID: 32459192.
- Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10(10):CD007498. PMID: 29025194; PMCID: PMC6485408.
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 Jan 16;397(10270):220-232. doi: 10.1016/S0140-6736(20)32656-8. PMID: 33428867; PMCID: PMC7833295.
- Carfi, A., Bernabei, R., Landi, F. & Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 324, 603–605 (2020).
- Chopra, V., Flanders, S. A. & O’Malley, M. Sixty-day outcomes among patients hospitalized with COVID-19. Ann. Intern. Med. https://doi.org/10.7326/M20-5661 (2020).
- Mathieu, E., Ritchie, H., Ortiz-Ospina, E. et al. A global database of COVID-19 vaccinations. Nat Hum Behav (2021).
- Paul Erlich Institute – Infographics. https://www.pei.de/EN/medicinal-products/vaccines-human/covid-19/covid-19-node.html. Visited on June 23rd, 2021.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars







