Printed from acutecaretesting.org
October 2009
D-dimer testing in the treatment and monitoring of septic patients
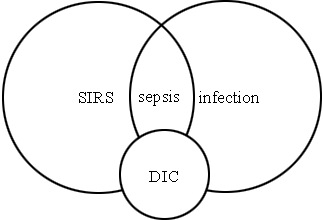
Sepsis is one of the leading causes of death in intensive care medicine increased nearly 10 % each year during the last 2 decades. Sepsis is an overwhelming primary local infection leading from systemic inflammatory response syndrome (SIRS) to shock and multiple organ failure (MOF) with a mortality rate of 60 %.
The main pillars in the treatment are local clearance, early effective antimicrobial therapy, supporting intensive care and special forms of organ support.
Infection leads to activation of inflammatory processes inevitably involving activation of coagulation.
An overwhelming inflammatory response may lead to organ failure, and the coagulation and fibrinolysis involvement may lead to disseminated intravascular coagulation (DIC), a pathological activation of coagulation that leads to the formation of small blood clots inside the blood vessels throughout the body.
FIGURE 1
A number of interactive systemic factors appear to promote the development of organ failure and cytokine-induced coagulation, and thrombin activates immune cells.
Coagulation activation is almost always present in infections and systemic host response, as indicated by elevated plasma levels of D-dimer, prothrombin fragments and thrombin-antithrombin complexes.
During sepsis, the regulatory mechanisms of blood coagulation are severely disturbed, resulting in fibrin deposition in the microcirculation, which is thought to be the major cause of organ failure.
Multiple studies have suggested that coagulation abnormalities may develop even before the onset of clinical symptoms of severe sepsis or septic shock.
Coagulopathy, as evidenced by the consumption of coagulation factors and suppression of the fibrinolytic system, results in the microvascular fibrin deposition responsible for multiple organ failure in severe sepsis.
PHYSIOLOGY REMARKS
Formation and dissolution of fibrin are key events in hemostasis. Physiologically, coagulation is initiated by the tissue factor – factor VIIa complex – which promotes thrombin generation subsequently converting fibrinogen to fibrin monomers.Active factor XIII links the so-called D-domains of fibrin monomers and thereby generates a solid fibrin clot.
Specific degradation of fibrin in monomeric and crosslinked form is achieved by the plasminogen system, which in turn is counterbalanced by inhibitors released by endothelial cells or platelets.
D-dimer, which is present in the degradation products, is not present in fibrinogen or the fibrin monomer. Therefore, plasma levels of D-dimer are specific for the plasmin-catalyzed degradation of the fibrin polymer.
Thereby D-dimer plasma levels specifically reflect the turnover of the coagulation system. Even in healthy subjects D-dimer plasma levels can be detected, indicating a certain level of fibrinogen-fibrin turnover under normal physiological conditions.
A blockade of the plasmatic coagulation by anticoagulants as well as a blockade of fibrinolysis using antifibrinolytics will therefore decrease D-dimer plasma levels.
SEPTIC PATIENT
In septic patients endothelial activation may lead to locally induced coagulation. A large variety of fibrin compounds can be detected in plasma from patients with intravascular coagulation activation.Almost all patients admitted with sepsis have elevated D-dimer levels very closely related to organ dysfunction and outcome.
In addition, changes of coagulation parameters within the first 2 days have a huge influence on the development of new or resolution of organ dysfunction and consequently on the mortality of septic patients [2].
This may indicate that early therapeutic interventions in a newly admitted septic patient are the most important ones and that early diagnosis and treatment of coagulopathy are necessary for septic patients.
PATIENTS WITH TRAUMA
Patients admitted to hospital after trauma generally have elevated D-dimer levels, indicating ongoing coagulation and fibrinolysis. During the course of stay D-dimer levels decline, indicating that there is no longer increased coagulation and fibrinolysis.DIAGNOSIS OF DISSEMINATED INTRAVASCULAR COAGULATION (DIC)
Within established scoring systems for organ dysfunction the presence of coagulation abnormalities or DIC only play minor roles: The most widely used APACHE II score combines surrogate markers of different organ failures with demographic data.
White-blood-cell count, body temperature, blood pressure or heart rate has no relevant influence on D-dimer levels in septic patients although all these parameters are part of the APACHE II score.
Coagulation or disseminated intravascular coagulation is not part of established scoring systems. This ignores the prognostic significance of coagulation activation processes in severely ill patients.
Multiple organ failure always involves coagulation activation, and ongoing DIC leads to purpura fulminans and bleeding due to consumption coagulopathy or microvascular thrombosis.
The combination of different organ dysfunction scoring systems with DIC scores may be a clinically applicable approach [5].
Several diagnostic criteria for disseminated intravascular coagulation (DIC) have been proposed, such as the DIC scoring system of the International Society of Thrombosis and Hemostasis (ISTH) for non-overt and overt DIC [6] or the JAAM DIC score [7,8].
According to these diagnostic criteria, four widely available parameters should be used: prothrombin time, platelet count, fibrinogen level and a fibrin-related marker. Possible fibrin-related markers are D-dimer plasma levels, fibrin degradation products, or soluble fibrin.
Both the overt and non-overt scoring systems have been prospectively validated, and this demonstrated the overt DIC scoring system to be sufficiently accurate to diagnose DIC in intensive care unit (ICU) patients [9].
It is useful to determine D-dimer plasma levels during the course of sepsis and septic shock: Continuation or worsening of coagulopathy during the first day of severe sepsis was associated with increased development of new organ failure and 28-day mortality.
SUCCESSFUL INTERVENTIONS
Mortality from sepsis correlates with the number of failing organs and the degree of organ dysfunction. Patients without organ failure have a mortality rate of approximately 15 % compared with 70 % for those with three or more failing organs.DIC can be seen as a separate organ failure, which might be prevented. Anticoagulation leads to lowering levels of D-dimer in patients with thromboembolic diseases.
It cannot be assumed that this intervention may be simply done in patients with septic DIC: If the coagulation system is already exhausted and bleeding occurs, no further coagulation inhibition seems to be effective.
The frequency of DIC in severe sepsis ranges between 40.7 % in the KyberSept trial (antithrombin) [10] and 22.4 % in the PROWESS study (recombinant activated protein C) [11].
Patients with increasing DIC severity usually have decreasing anticoagulant activities as shown in 257 own patients.
FIGURE 2: Increasing DIC score and antithrombin activity in septic patients
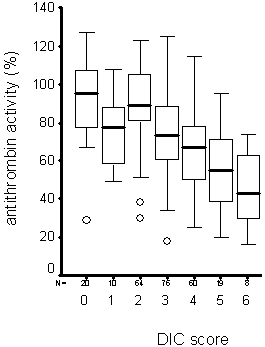
Various studies in patients with DIC clearly showed that a decrease in DIC severity is associated with beneficial outcome, whereas a worsening DIC to overt DIC is associated with organ failure and death.
Therefore early interventions with anticoagulants like heparin, antithrombin, activated protein C or tissue factor pathway inhibitors might be useful for improving the mortality in patients with DIC.
The most efficient treatment might be achieved in patients at risk before the development of DIC than in the already fully overt DIC patients.
In a newly diagnosed septic patient changes during the first day of treatment had the highest impact on mortality, development and resolution of organ failure.
The activated protein C study in patients with severe sepsis [11] was the first study demonstrating a possible proof of the principle: Intervention in the coagulation pathways clearly leads to a change in D-dimer plasma level.
This was associated with a better outcome. The study was, however, not intended to study patients with severe sepsis and with DIC.
FUTURE
Using scoring systems for both organ dysfunction and DIC may separate patients who will most likely benefit from anticoagulant therapy from those in whom the coagulation must be strengthened.Combining the composite coagulopathy score with the APACHE II score improved the ability to identify patients who would progress to multiple organ failure [2].
CONCLUSION
Anticoagulant agents might be useful for improving the mortality in patients with DIC, especially during the early phase of DIC. The initiation of DIC treatment based on the new diagnostic DIC criteria may reduce the mortality rate of patients with DIC.
One major component in the laboratory assessment of DIC is D-dimer, which is suggested to be monitored from day 1 until resolution of septic insult.
References+ View more
- Angstwurm MW, Reininger AJ, Spannagl M. D-dimer as marker for microcirculatory failure: correlation with LOD and APACHE II scores. Thromb Res 2004; 113: 353-59
- Dhainaut JF. Dynamic evolution of coagulopathy in the first day of severe sepsis: Relationship with mortality and organ failure. Crit Care Med 2005; 33: 341-48
- Iba T, Gando S, Murata A, Kushimoto S et al. Predicting the severity of SIRS-associated coagulopathy with hemostatic molecular markers and vascular endothelial injury markers. J Trauma 2007; 63:1093-98
- Sawamura A, Hayakawa M, Gando S, Kubota N, Sugano M, Wada T, Katabami KI. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thromb Res 2009; August 4
- Angstwurm MW, Dempfle CE, Spannagl M. New disseminated intravascular coagulation score: A useful tool to predict mortality in comparison with Acute Physiology and Chronic Health Evaluation II and Logistic Organ Dysfunction scores. Crit Care Med 2006; 34: 314-20
- Taylor FB, Toh CH, Hoots WK, et al. Towards Definition, Clinical and Laboratory Criteria, and a Scoring System for Disseminated Intravascular Coagulation. Thromb Haemost 2001; 86: 1327-30
- Gando S, Iba T, Eguchi Y, et al: A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria. Crit Care Med 2006; 34: 625-31
- Gando S, Saitoh D, Ogura H et al: Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med 2008; 36: 145-50
- Bakhtiari K, Meijers JCM, de Jonge E et al: Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med 2004; 32: 2416-21
- Warren BL, Eid A, Singer P et al: Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 2001; 286: 1869-78
- Bernard GR, Vincent JL, Laterre PF et al: Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001; 344: 699-709
References
- Angstwurm MW, Reininger AJ, Spannagl M. D-dimer as marker for microcirculatory failure: correlation with LOD and APACHE II scores. Thromb Res 2004; 113: 353-59
- Dhainaut JF. Dynamic evolution of coagulopathy in the first day of severe sepsis: Relationship with mortality and organ failure. Crit Care Med 2005; 33: 341-48
- Iba T, Gando S, Murata A, Kushimoto S et al. Predicting the severity of SIRS-associated coagulopathy with hemostatic molecular markers and vascular endothelial injury markers. J Trauma 2007; 63:1093-98
- Sawamura A, Hayakawa M, Gando S, Kubota N, Sugano M, Wada T, Katabami KI. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thromb Res 2009; August 4
- Angstwurm MW, Dempfle CE, Spannagl M. New disseminated intravascular coagulation score: A useful tool to predict mortality in comparison with Acute Physiology and Chronic Health Evaluation II and Logistic Organ Dysfunction scores. Crit Care Med 2006; 34: 314-20
- Taylor FB, Toh CH, Hoots WK, et al. Towards Definition, Clinical and Laboratory Criteria, and a Scoring System for Disseminated Intravascular Coagulation. Thromb Haemost 2001; 86: 1327-30
- Gando S, Iba T, Eguchi Y, et al: A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria. Crit Care Med 2006; 34: 625-31
- Gando S, Saitoh D, Ogura H et al: Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med 2008; 36: 145-50
- Bakhtiari K, Meijers JCM, de Jonge E et al: Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med 2004; 32: 2416-21
- Warren BL, Eid A, Singer P et al: Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 2001; 286: 1869-78
- Bernard GR, Vincent JL, Laterre PF et al: Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001; 344: 699-709
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Register for the related webinar
The Benefit of Using a D-dimer Assay with a High Clinical Specificity
Presented by Karin Strandberg, MD, PhD, Assoc. Prof.
Join the webinarAcute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars