Printed from acutecaretesting.org
September 2004
Diabetic ketoacidosis
Diabetes mellitus is the name given to a group of conditions whose common hallmark is a raised blood glucose concentration (hyperglycemia) due to an absolute or relative deficiency of the pancreatic hormone insulin.
In the UK there are 1.4 million registered diabetic patients, approximately 3 % of the population. In addition, an estimated 1 million remain undiagnosed. It is a growing health problem: In 1998, the World Health Organization (WHO) predicted a doubling of the worldwide prevalence of diabetes from 150 million to 300 million by 2025.
For a very tiny minority, diabetes is a secondary feature of primary endocrine disease such as acromegaly (growth hormone excess) or Cushing’s syndrome (excess corticosteroid), and for these patients successful treatment of the primary disease cures diabetes. Most diabetic patients, however, are classified as suffering either type 1 or type 2 diabetes.
Type 1 diabetes
Type 1 diabetes, which accounts for around 15 % of the total diabetic population, is an autoimmune disease of the pancreas in which the insulin-producing β-cells of the pancreas are selectively destroyed, resulting in an absolute insulin deficiency.The condition arises in genetically susceptible individuals exposed to undefined environmental insult(s) (possibly viral infection) early in life.
It usually becomes clinically evident and therefore diagnosed during late childhood, with peak incidence between 11 and 13 years of age, although the autoimmune-mediated β-cell destruction begins many years earlier.
There is currently no cure and type 1 diabetics have an absolute life-long requirement for daily insulin injections to survive.
Type 2 diabetes
This is the most common form of diabetes: around 85 % of the diabetic population has type 2 diabetes. The primary problem is not decreased insulin production but reduced ability of tissue cells to respond to normal amounts of insulin; this is termed insulin resistance.Initially the pancreas is able to respond to insulin resistance and increase insulin production, but eventually this compensatory increase is insufficient to overcome insulin resistance and hyperglycemia, the hallmark of diabetes, intervenes.
In the long term, reduced insulin production by the pancreas is a contributory factor. There is a family history of diabetes among most patients diagnosed with type 2 diabetes, highlighting a strong genetic component in the etiology of the condition.
Obesity and lack of physical exercise are significant predisposing environmental factors. Type 2 diabetes is predominantly an adult disease, usually presenting in middle or old age.
In recent years, however, with the rise in childhood obesity there are increasing reports of type 2 diabetes being diagnosed during childhood. Treatment of type 2 diabetes is based initially on dietary advice with the twin aims of carbohydrate restriction and weight reduction.
Drugs which lower blood glucose (oral hypoglycemics) may also be necessary. In a minority of cases regular insulin injections may in the long term be prescribed if dietary adjustment and oral hypoglycemic therapy fail to normalize blood glucose concentration sufficiently.
Diabetic ketoacidosis – an acute complication of diabetes
The chronic long-term complications of prolonged hyperglycemia, which occur among diabetics who remain undiagnosed or among diagnosed patients whose therapy fails to adequately control blood glucose concentration, include diabetic retinopathy (eye disease, which can result in blindness), diabetic nephropathy (kidney disease) and diabetic neuropathy (loss of peripheral nerve function).
In addition, diabetics are at increased risk of cardiovascular disease.
Diabetic ketoacidosis (DKA), by contrast, is an acute complication of diabetes which evolves over a period of less than 24 hours and results directly from insulin deficiency. It is a medical emergency.
A recent survey revealed that 9 % of type 1 diabetics suffered an episode of DKA at least once during the previous 12 months. Before the advent of insulin therapy in the 1920s, premature death due to DKA was the fate of the majority of diabetics.
Although now thankfully rare, deaths due to DKA continue to occur, most often among young children and the elderly.
Overall mortality due to DKA is between 2 and 5 % in countries like the UK, where emergency medical care is readily available.
DKA can be the first sign of diabetes but most (80 %) cases occur in previously diagnosed patients. A precipitating cause for DKA can be identified in 80 % of cases. Failure to take insulin and intercurrent illness, notably infection, are the most usual causes.
Although DKA is predominantly a complication of type 1 diabetes, major surgery, severe illness such as myocardial infarction, or severe infection (sepsis) can precipitate an episode of DKA in type 2 diabetic patients because these conditions are associated with increase in stress hormones (cortisol, catecholamines) which oppose the action of insulin, thereby exacerbating the relative insulin deficiency that defines type 2 diabetes.
Since the primary defect in DKA is severe insulin deficiency, an understanding of the pathogenesis of DKA demands, at the very least, outline knowledge of the normal action of insulin.
Normal action of insulin
Insulin is produced in and secreted from the β-cells of the pancreas in response to a rising blood glucose concentration. The result of insulin action is reduction in blood glucose concentration; as blood glucose concentration falls, insulin production and secretion are stepped down.
The reduction of blood glucose concentration occurs because of two effects of insulin. Firstly, insulin promotes uptake of glucose from blood by tissue cells (in tissue cells throughout the body glucose is metabolized (oxidized) to provide energy).
Secondly, insulin inhibits the liver production of glucose from non-carbohydrate sources and promotes the storage of glucose as glycogen.
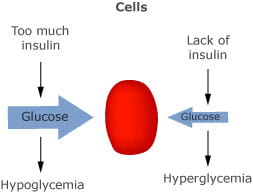
FIG 1. Lack of insulin inhibits entry of glucose to cells with resulting hyperglycaemia
Apart from its effect on blood glucose concentration, insulin has an important effect on fat metabolism in that it inhibits the enzyme lipase, an enzyme essential for the breakdown of triglyceride (stored fat) to its constituent fatty acids, a step necessary for the potential energy in fat to be realized.
The negative effect of insulin on fat metabolism is appropriate because glucose is available as an energy source. In periods of fasting or starvation when blood glucose is low and insulin production is consequently switched off, fat is metabolized as an alternative energy source.
There are several hormones, the so-called counterregulatory hormones, which oppose the action of insulin; the most important of these is glucagon (also produced within the pancreas but, by contrast to insulin, in response to a falling blood glucose).
Glucagon increases blood glucose by promoting the liver production of glucose from non-carbohydrate sources and stored glycogen. Other hormones which oppose the action of insulin include the stress hormones produced by the adrenal glands (cortisol and catecholamines) growth hormone and estrogen.
By integration of the combined action of insulin and the counterregulatory hormones, blood glucose is maintained within narrow limits and cells continue to be supplied with a sufficient source of potential energy to function normally.
Pathogenesis of DKA – the effect of severe insulin deficiency
In the absence of insulin, glucose in blood cannot enter tissue cells where it is needed to provide energy, and the liver inappropriately continues to release glucose produced from non-carbohydrate sources and breakdown of glycogen to the blood.
The inevitable consequence is a rising blood glucose concentration. DKA is often described as ‘starvation in the midst of plenty’ because tissue cells deficient of their much needed energy source, glucose, are bathed by extracellular fluid increasingly rich in glucose.
Most of the clinical signs and symptoms which characterize DKA can be attributed to either raised blood glucose (hyperglycemia) or lack of glucose within cells.
The abnormalities in results of blood gas analysis stem mainly from lack of glucose within cells but first, for completeness, the consequences of hyperglycemia will be addressed.
Consequences of hyperglycemia in DKA
At normal blood glucose concentration, tubular reabsorption in the kidney ensures that virtually no glucose is excreted in urine. This mechanism is overwhelmed when blood glucose concentration rises, and glucose appears in urine.Since glucose is an osmotically active substance, this loss of glucose in urine is associated with an osmotic diuresis and resulting dehydration, as an increasing volume of water is lost from the body in urine.
Typically, a total body water deficit of 5-7 liters, around 10 % of body weight, occurs in DKA. Rising plasma osmolarity due to dehydration invokes the thirst response to correct the water deficit.
By this sequence of events, raised blood glucose gives rise to four classical signs/symptoms of DKA: glycosuria (abnormal amount of glucose in urine), polyuria (increased urine volume), dehydration and thirst.
The water deficit reduces blood volume, thereby decreasing blood pressure, so that hypotension is a presenting feature of DKA. Osmotic diuresis is associated with large losses of electrolytes in urine, so that patients with DKA typically have a whole-body sodium and potassium deficit of 500-700 mmol and 200-300 mmol, respectively.
If the fluid losses remain uncorrected, reduced renal blood flow consequent on reduced blood volume threatens renal function. This in turn reduces renal excretion of glucose, thereby exacerbating hyperglycemia and all its deleterious consequences.
In extreme cases, patients may present in acute renal failure. In summary, hyperglycemia in DKA causes an osmotic diuresis, which results in severe fluid and electrolyte deficit.
If left untreated, fluid deficit can be sufficiently severe to cause acute renal failure.
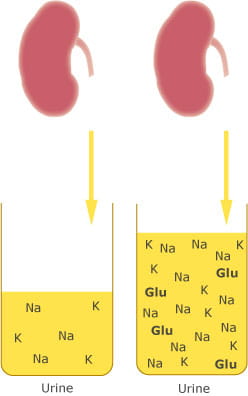
FIG 2. Osmotic diuresis in DKA causes polyuria, glycosuria and electrolyte depletion.
Consequences of intracellular glucose deficiency – ketoacidosis
The abnormalities seen in the results of blood gas analysis among patients suffering DKA are a result of the metabolic changes that occur because cells are deficient of their normal source of energy, glucose.These metabolic changes are an extension of the normal physiological response to starvation, when glucose is also in short supply, in this case not because of insulin deficiency, but because dietary carbohydrate, from which glucose is derived, is restricted.
When glucose is in short supply, an alternative energy source is provided for in stored fat, and it is this switch to fat metabolism which lies at the root of the disturbance of acid-base balance in patients with DKA.
Fat is stored as triglycerides in adipocytes, the specialized cells of which adipose (fat) tissue is composed. The mobilization of fat as an alternative energy source begins with the breakdown (lipolysis) of triglycerides to its constituent free fatty acids and glycerol by the hormone-sensitive enzyme, lipase.
Insulin inhibits lipolysis, whilst the counterregulatory hormones promote lipolysis. In patients with DKA, therefore, lipolysis and the resulting production of free fatty acids proceed relatively unhindered.
Free fatty acids are transported, bound to albumin, from adipocytes to tissues around the body where they are oxidized in cell mitochondria to acetyl CoA, providing much needed energy in the form of adenosine triphosphate (ATP) in the process.
Acetyl CoA is further metabolized to carbon dioxide and water in the citric acid cycle, yielding more energy-rich ATP. The liver provides an alternative fate for acetyl CoA if, as is the case in severe insulin deficiency, its production exceeds the metabolic capacity of the citric acid cycle.
In this case, the liver converts some of the acetyl CoA derived from oxidation of fatty acids to the ketoacid, acetoacetic acid. Some of this acetoacetic acid is converted to a second ketoacid, β-hydroxybutyric acid, and acetone.
Collectively, the two ketoacids and acetone are known as ketone bodies or simply ketones. They are released from the liver to blood and delivered to peripheral tissues where the two ketoacids are utilized as energy substrates.
Excess ketones are excreted in urine. Expired air provides an additional route for excretion of volatile acetone, giving rise to the characteristic ‘nail varnish remover’ smell of acetone, on the breath of patients with DKA.
The problem in DKA is that the rate at which ketones are produced exceeds the rate at which they can be metabolized/excreted and they accumulate in blood with disastrous results.
Accumulation of ketoacids overwhelms the normal buffering capacity of blood
The pH of blood (ECF) is normally maintained within very narrow limits (7.35-7.45) by a complex synergy of action involving the lungs, kidneys and bicarbonate buffering system in blood.Normal metabolism is associated with continuous production of hydrogen ions (H+) which tend to reduce pH, but bicarbonate in blood (ECF) combines with these hydrogen ions forming carbonic acid:
H+ +
HCO-3 ![]() H2CO3
H2CO3
(bicarbonate)
(carbonic acid)
This is then converted to carbon dioxide and water:
H2CO3 ![]() H2O + CO2
H2O + CO2
Carbon dioxide is eliminated from the body by the lungs in expired air and bicarbonate is regenerated in the kidneys.
By this means, the concentration of hydrogen ions in blood, and therefore the pH, remains constant despite continuous production of metabolic acids. The abnormal accumulation of ketoacids in blood that occurs in DKA overwhelms this buffering system.
Both ketoacids are strong acids and dissociate completely at physiological pH:
CH3COCH2COOH ![]() CH3COCH2COO- +
H+
CH3COCH2COO- +
H+
(acetoacetic
acid)
(acetoacetate)
CH3CH(OH)CH2
COOH ![]() CH3CH(OH)CH2
COO- + H+
CH3CH(OH)CH2
COO- + H+
β-hydroxybutyric
acid)
(β-hydroxybutyrate)
yielding hydrogen ions. The rate at which bicarbonate can be regenerated cannot keep pace with the rate at which it is being used to buffer this abnormal influx of hydrogen ions.
Eventually, a point is reached when buffering fails and hydrogen ion concentration increases, and pH falls below normal. The patient is said to be suffering a metabolic acidosis, and blood gas analysis at this stage reveals reduced pH in combination with a reduced bicarbonate concentration.
In severe DKA, pH may fall below 7.0 and bicarbonate below 10 mmol/L (approximate normal range 25-30 mmol/L).
The maintenance of a normal blood pH is crucial for life and the body has compensatory mechanisms which serve to return an abnormal pH towards normal during acid-base disturbances, whatever the cause.
To understand the compensatory mechanism which is invoked during DKA, it is useful to recall a simple relationship that defines pH in terms of the concentration of bicarbonate and carbon dioxide in blood:
pH ![]() [HCO3] / pCO2
[HCO3] / pCO2
This relationship states that the pH of blood is proportional to the ratio of bicarbonate concentration to partial pressure of carbon dioxide (pCO2). During DKA, pH is low primarily because the bicarbonate buffer is exhausted, i.e. bicarbonate concentration is reduced.
The above relationship dictates that if pCO2 were reduced by relatively the same amount, then pH would return to normal. Carbon dioxide is eliminated from the body by the lungs and a reduction in pCO2 is achieved by increasing the rate of respiration (i.e. hyperventilating).
So the compensatory mechanism invoked by DKA is increased respiration. The deep and rapid sighing respiration, called Kussmaul's respiration, in patients suffering DKA provides clinical evidence of this compensatory mechanism.
The decreased pCO2 that results from this increased respiration returns the pH towards normal but may not be sufficient to achieve a normal pH.
The patient is said to be either fully compensated if pH is within the normal range, or partially compensated if pH remains below the lower limit of normal.
A summary of the typical results of blood gas analysis in DKA are provided in Table I.
|
Diabetic ketoacidosis |
||||
|
Normal range |
Mild |
Moderate |
Severe |
|
|
pH |
7.35-7.45 |
7.25-7.30 |
7.00-7.24 |
<7.00 |
|
pCO2 (kPa) |
4.7-6.0 |
3.6-4.0 |
2.8-3.6 |
1.8-2.8 |
|
pCO2 (mmHg) |
35-45 |
27-30 |
21-27 |
13.5-21 |
|
Bicarbonate |
22-28 |
15-18 |
10-15 |
<10 |
Typical blood gas results in untreated diabetic ketoacidosis
Acidosis causes nausea and vomiting, a frequent presenting symptom of DKA. Loss of fluid in vomit exacerbates the water deficit and consequent dehydration induced by osmotic diuresis.
Acidosis is a contributory factor in the development of the dangerously high plasma potassium (hyperkalemia) that is also a feature of untreated DKA, because acidosis induces an exchange of hydrogen ions for potassium ions across cell membranes with hydrogen ions passing into cells and potassium ions passing out of cells to the ECF (blood plasma).
Hyperkalemia, however, masks the underlying whole-body potassium deficiency that occurs in DKA.
Principles of DKA treatment
Administration of insulin, replacement of lost fluid with intravenous infusion of isotonic saline and replacement of potassium losses are the three main features of DKA treatment.
The inhibitory effect of insulin on lipolysis is immediate and highly effective in halting ketoacid production and thereby normalizing acid-base balance.
In extreme acidosis (pH < 7.0), the use of bicarbonate infusion is sometimes used to help correct the acidosis.
Careful and intensive monitoring of blood biochemistry (particularly blood glucose, potassium and pH) and fluid balance is essential for a favorable outcome.
|
Signs and symptoms:
Urine analysis reveals:
Blood testing reveals:
|
TABLE II. Clinical features of diabetic ketoacidosis
SUMMARY
Diabetic ketoacidosis is the extreme and life-threatening metabolic derangement that results from severe insulin deficiency.The disturbance of normal acid-base balance, revealed by blood gas analysis, which is central to its pathogenesis, gives rise to many of the signs and symptoms (Table II) that characterize the condition.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









