Printed from acutecaretesting.org
July 2007
Ionized calcium
Introduction
The adult human body contains 1-1.3 kg of calcium. Almost all (99 %) of this is contained within bones and teeth. The remaining 1 % is distributed between soft-tissue cells and extracellular fluid (i.e. interstitial fluid and blood plasma). Just 8.7 mmol (350 mg) calcium circulates in blood plasma at a concentration of around 2.5 mmol/L (10 mg/dL).
Of this 350 mg around 40 % is bound to protein (predominantly albumin, but also globulins) and 10 % is complexed with a range of anions (bicarbonate, lactate, phosphate, etc). The remaining 50 % circulates as "free" ionized calcium (Ca2+) at a concentration of around 1.25 mmol/L (Fig. 1).
The three fractions of calcium present in blood plasma are in equilibrium, but crucially only the ionized calcium fraction is physiologically active [1,2]. A small proportion ( » 1 %) of the calcium in bone is readily exchangeable with plasma.
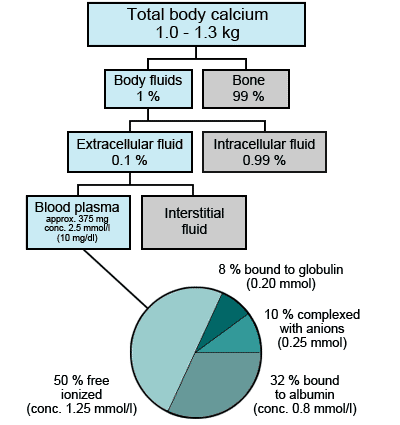
FIGURE 1: Distribution of calcium in the body
Functions of calcium
Most of the calcium in bone is present with phosphate as hydroxyapatite [Ca10(PO4)6OH2] crystals. Hydroxyapatite is a major structural component of bone, accounting for 65 % of bone weight. In addition to its major structural function, the calcium in bone is a reservoir of calcium that is important for maintaining plasma calcium concentration.
The ionized calcium present in blood plasma is a co-factor for two of the enzymes (blood-clotting factors) involved in the production of fibrin by the clotting cascade, so that calcium is essential for the process of blood coagulation. The action of many other enzymes – both extra- and intracellular – depends on ionized calcium as co-factor.
Physiological processes that depend on ionized calcium include myocardial, skeletal and smooth muscle contraction, neural conduction and synaptic signaling (neuromuscular transmission). Ionized calcium acts as a second messenger in the process that results in hormone secretion by endocrine tissue cells. In fact, a range of intracellular processes, including cell division, depend on calcium signaling.
In the absence of minute (nanomolar) quantities of intracellular ionized calcium, these processes, essential for cellular integrity and function, could not occur.
Calcium homeostatis
The concentration of calcium in blood plasma reflects a balance between the calcium absorbed from diet via the gastrointestinal tract and that lost from the body in urine. In addition, calcium can move between plasma and bone. (The critical importance of maintaining plasma calcium concentration within normal limits is reflected in the fact that if calcium is in short supply, the body will sacrifice bone mineralization to maintain plasma calcium concentration).
Gastrointestinal absorption of dietary calcium, renal excretion of calcium and the movement of calcium out of bone, the three main determinants of plasma calcium concentration are all under hormonal control. The principal hormones involved are parathyroid hormone and calcitriol (the active metabolite of vitamin D) [3].
Parathyroid hormone (PTH) is produced in and secreted from the four tiny (rice-grain sized) parathyroid glands situated in the neck, in response to falling plasma ionized calcium concentration. A transmembrane calcium-sensing receptor present on the surface of parathyroid cells mediates this response [4]. The principle target organs of PTH are bone and the kidneys. By its action on osteoclasts, PTH promotes resorption of bone and thereby movement of calcium from bone to blood.
In the renal tubules, PTH promotes calcium reabsorption to blood. The net effect of PTH, then, is reduced calcium in bone, reduced excretion of calcium and increased plasma calcium concentration. As these changes occur, the stimulus to PTH secretion – reduced ionized calcium – is removed, and PTH secretion is stepped down (Fig. 2).
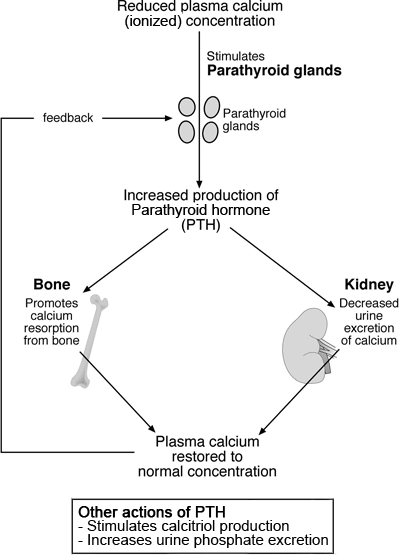
FIGURE 2: Parathyroid control of plasma calcium
Calcitriol (alternative name 1,25-dihydroxycalciferol) is produced in the kidneys from a vitamin D-derived substance, 25-hydroxycholecalciferol. PTH stimulates the enzymatic conversion of 25-hydroxycholecalciferol to calcitriol. In common with PTH, calcitriol secretion is promoted by reduced plasma ionized calcium concentration and inhibited by raised plasma ionized calcium concentration.
The principal target tissue of calcitriol is the gastrointestinal tract where it promotes absorption of dietary calcium. The effect, then, is to raise plasma calcium concentration.
By the integrated action of PTH, calcitriol and the negative feedback effect of ionized calcium concentration on release of these hormones, ECF (plasma) calcium concentration is maintained within normal limits.
To summarize, maintenance of normal plasma calcium concentration depends on:
- diet containing adequate amounts of calcium
- exposure to sunlight for endogenous production of vitamin D plus dietary source of vitamin D (both are required for optimum calcitriol production)
- normal gastrointestinal function for absorption of dietary calcium and vitamin D
- normal parathyroid function for appropriate secretion of PTH
- normal renal function for secretion of calcitriol, and appropriate adjustment of calcium loss in urine
- normal bone metabolism for appropriate movement of calcium between bone and blood
Assessing calcium status – the rationale for measuring ionized rather than total plasma calcium concentration
For well over 50 years the most common method of assessing calcium status has been to measure concentration of total calcium (i.e. the sum of protein-bound, complexed and free ionized calcium) in plasma or serum [5]. The colorimetric methods that continue to be used to measure total calcium are robust and available on all major clinical chemistry automated platforms.
There are few potential preanalytical errors associated with measurement of total calcium, and the blood sample used for routine biochemistry profiling can also be used for total calcium measurement. In addition to the great ease and convenience of measuring total calcium, the test has the advantage of familiarity; clinicians feel comfortable interpreting results [6].
For as long as calcium has been routinely measured in clinical laboratories, it has been appreciated that the physiological activity of calcium resides in the ionized fraction of total calcium and that this is the fraction that is clinically significant [7]. The clinical validity of total calcium measurement is based on the assumption that total calcium concentration accurately reflects ionized calcium activity. Whilst that is the case in healthy individuals, it is not necessarily the case in some sick individuals.
The two principal clinical situations in which total calcium concentration does not sufficiently accurately reflect ionized calcium activity are:
- those in which the patient's serum protein concentration is abnormal
- those in which the patient is suffering disturbance of the acid-base balance
The significance of serum protein concentration lies in the observation that the amount of calcium bound by serum proteins is directly proportional to protein concentration. If serum protein concentration increases, then the concentration of protein-bound calcium and therefore total calcium concentration increases. Conversely, if plasma protein concentrations decrease, total plasma calcium decreases.
The important point is that despite an increase or decrease in measured total calcium concentration, ionized calcium concentration, the physiologically and clinically important parameter, remains essentially unchanged.
Since most of the protein-bound calcium in serum is bound to albumin, it is change in serum albumin concentration that is most significant in affecting total calcium concentration. Interpretation of total calcium results should always include due consideration of serum albumin concentration.
To mitigate the effect of abnormal serum protein concentration on total calcium measurement, a number of formulae have been devised for estimation of "corrected" total calcium concentration from measured total calcium and either serum albumin or protein concentration [8-11]. One of the most widely used ones in the US is the so-called "modified" Orrell correction [1]:
Corrected calcium (mg/dL) = measured total calcium (mg/dL) + 0.8 (4 – serum Alb g/dL)
Whichever formula is used, "corrected" calcium is an estimate of the total calcium concentration, had serum protein (albumin) concentration been normal. Although "corrected" total calcium reflects ionized calcium more accurately than uncorrected total calcium in patients with abnormal serum protein concentration, none of the correction formulae are entirely reliable for all patients.
This is because the formulae do not, nor could they, take account of all the many factors that can affect calcium binding in particular patients. The limitations of the correction formulae have been exposed by a number of studies that have focused principally, although not exclusively, on the critically ill [12-15].
One recent representative study [16] revealed that "corrected" calcium failed to accurately classify calcium status (hypocalcemia, normocalcemia or hypercalcemia) in 38 % of 110 intensive care patients. In common with other studies, "corrected" serum calcium was found to underestimate the prevalence of hypocalcemia and overestimate the prevalence of normocalcemia.
Given that hypoalbuminemia is a common feature of critical illness and that none of the correction factors applied to total calcium results are reliable in predicting ionized calcium concentration in this patient group [15], measurement of total calcium is considered an inappropriate measure of calcium status among the critically ill. For this patient population, at least, the only reliable means of assessing calcium status is to measure ionized calcium directly.
Quite apart from the problem of abnormal serum protein concentration, serum/plasma total calcium does not accurately reflect ionized calcium activity in patients suffering disturbance of acid-base. Blood pH is a major determinant of the proportion of total calcium that is bound to protein, principally because hydrogen ions compete with calcium ions for protein binding sites [17].
A decreased pH (acidosis) is associated with decreased calcium binding and therefore increased proportion of total calcium in the ionized state. To give some idea of the magnitude of this effect, each 0.1 decrease in pH results in a 0.05 mmol/L increase in serum ionized calcium concentration [18]. By the same mechanism, raised blood pH (alkalosis) causes reduction in serum ionized calcium concentration.
Since this phenomenon is merely a shift of calcium from one fraction to another, total calcium concentration is not affected. Despite what may well be a clinically significant change in calcium status, serum total calcium concentration remains unchanged. The most reliable way of assessing calcium status among patients with concomitant acid-base disturbance is to measure ionized calcium directly.
Ionized calcium has been shown to be more reliable than total corrected calcium in some other patient groups, including those suffering mild hyperparathyroidism [19] and those with renal failure [20].
Abnormality in serum/plasma ionized calcium concentration
In health, plasma ionized calcium concentration is maintained between approximately 1.15 and 1.30 mmol/L. Hypercalcemia (increased amount of calcium in blood), diagnosed if ionized calcium is >1.30 mmol/L, is more common than hypocalcemia (reduced amount of calcium in blood). However, among certain patient groups, most notably the critically ill and neonates, hypocalcemia is the more common derangement.
Causes of hypercalcemia (ionized calcium > 1.30 mmol/L)
The two most common causes of hypercalcemia are primary hyperparathyroidism and malignant disease (cancer).
Primary hyperparathyroidism, the single most common cause accounting for around 50 % of all cases of hypercalcemia [21], is excessive uncontrolled secretion of PTH by a benign tumor (adenoma) in one of the four parathyroid glands. In a tiny minority, excessive PTH is due to abnormal increase in size (hyperplasia) of all parathyroid glands or parathyroid cancer.
The condition can occur at any age and in both sexes, but postmenopausal women are the most commonly affected. Excessive PTH secretion leads to bone demineralization (osteoporosis) and chronic hypercalcemia that predisposes to urine-stone formation and renal damage. In rare cases, the hypercalcemia is of sufficient severity to threaten life [22]. Surgical removal of the offending adenoma is curative.
Hypercalcemia can be a complication of soft-tissue cancer, most commonly cancers of breast, lung and esophagus. Hypercalcemia is also a common feature of multiple myeloma, a hematological malignancy of plasma cells in bone marrow. Taken together, malignant disease is the second most common cause of hypercalcemia.
One of the principal causes of hypercalcemia in these cases is uncontrolled excessive production by tumor cells of a protein called parathyroid hormone-related peptide (PTHrP) [23]. This is, as its name implies, similar to PTH in both structure and action. Like PTH, it increases plasma calcium by resorbing bone and decreasing calcium excretion. Unlike PTH, however, secretion of PTHrP is not inhibited by increasing ionized calcium concentration.
The uncontrolled action of PTHrP inevitably results in abnormal loss of calcium from bone and consequent hypercalcemia. Direct destruction of bone tissue (osteolysis) by tumor cells that have metastasized to bone can also result in hypercalcemia; this is the principal mechanism of the hypercalcemia associated with multiple myeloma.
Generally speaking, hypercalcemia develops late in malignant disease and is a poor prognostic sign [24]. It is still important to detect because treatment aimed at normalizing calcium provides relief from symptoms of hypercalcemia, which in turn materially improves the quality of life of affected cancer patients [25].
Taken together, primary hyperparathyroidism and malignant disease account for most (around 90 %) of cases of hypercalcemia. Rare causes of hypercalcemia [26] include chronic renal failure, hyperthyroidism, sarcoidosis and tuberculosis. Some drugs, including thiazide diuretics and lithium, can precipitate hypercalcemia, as can ingestion of excessive vitamin D.
Causes of hypocalcemia (ionized calcium < 1.15 mmol/L)
Hypocalcemia is a common feature of chronic renal failure, reflecting the important role of the kidneys in calcitriol production and, thereby, absorption of dietary calcium. Other causes of hypocalcemia include vitamin D deficiency and hypoparathyroidism (reduced PTH secretion by parathyroid glands), due most often to inadvertent destruction of parathyroid tissue during surgery of the neck or congenital lack of parathyroid glands.Hypocalcemia is much less common than hypercalcemia [27] except in two patient groups: the critically ill and neonates. Up to 85 % of patients being cared for in adult intensive care units develop hypocalcemia [28]. The conditions most frequently associated with hypocalcemia in this patient group are sepsis, acute pancreatitis, acute renal failure, severe burns, trauma with rhabdomyolysis, alkalosis and massive blood transfusion.
Hypocalcemia is relatively common in the neonatal intensive care unit. The normal transition from an intrauterine environment to physiological independence at birth includes a rapid reduction in plasma calcium concentration. In some babies, most notably the premature, those with low birth weight and those born to diabetic mothers, this physiological reduction is exaggerated and transient hypocalcemia develops due to inadequate PTH response of immature parathyroid glands [29].
Symptoms and consequences of hypercalcemia
In general, the range and severity of symptoms associated with hypercalcemia reflect the severity of the increase. Mild hypercalcemia, roughly defined as ionized calcium in the range 1.3 -1.50 mmol/L, may be asymptomatic, whereas severe hypercalcemia (ionized calcium > 1.70 mmol/L) is always associated with symptoms.
These may include gastrointestinal (nausea, vomiting, constipation) and neuropsychiatric (lethargy, depression, confusion); psychosis, seizures and coma may ensue. Myocardial contractility is affected, with arrhythmias and ECG changes (reduced QT interval). Cardiac arrest can occur if hypercalcemia is particularly severe. The effect of hypercalcemia on renal function is manifest acutely as polyuria and resulting polydipsia (thirst).
Long-standing (chronic) hypercalcemia, even if mild, predisposes to urine-stone formation and calcium-induced damage to renal tubule cells that can progress to renal failure.
Mild hypocalcemia, roughly defined as ionized calcium in the range 0.90-1.15 mmol/L, is often asymptomatic. Most common signs and symptoms associated with more severe hypocalcemia are manifestations of neuromuscular irritability, including paresthesia of peripheral extremities, muscle cramps, tetany and seizures.
Trousseau’s and Chvostek’s signs may be positive. Laryngeal spasm may restrict normal respiration and the effect on cardiac contractility may be evident as arrhythmias; ECG changes include prolonged QT interval and T-wave inversion. Markedly severe hypocalcemia can cause cardiac arrest. Long-standing hypocalcemia is associated with risk of cataracts.
Measurement of ionized calcium - sampling requirements
Measurement of ionized calcium is made using calcium ion-selective electrode (ISE) direct potentiometry. A reference method [30] has been described that is the basis of all routine methods available in blood gas and electrolyte analyzers. All that is required of the operator is introduction of sample (whole blood, serum or plasma) into the analyzer; results are available within a minute or two.
Despite the consensus that ionized calcium, rather than total calcium, is the preferred measure of calcium status and that ionized calcium measurement is simpler and faster, total calcium continues to be measured in clinical laboratories. Ionized calcium measurement has in general been confined to point-of-care settings such as recovery room, intensive care, emergency rooms and operating theaters. One of the reasons for the slow adoption of ionized calcium is that blood collection and preservation requirements are far more exacting than those required for measurement of total calcium.
It is vital that the pH of a blood sample for ionized calcium estimation is preserved because calcium binding and therefore ionized calcium concentration is pH dependant. For this reason, blood must be collected anaerobically to minimize the in vitro decrease in pH that would result from aerobic metabolism. If there is to be delay of more than 30 minutes before measurement, the sample must be chilled to +4 °C in ice slurry to further minimize cell metabolism. At this temperature whole-blood samples can be stored for up to 4 hours.
For whole-blood estimation, the most suitable if results are required urgently, the sample must be collected into a syringe containing the anticoagulant heparin in a lyophilized (dried) state. The use of standard lithium heparin is associated with significant potential error because heparin binds calcium, leading to artefactually reduced ionized calcium concentration.
The magnitude of this error is dependant on heparin concentration. To minimize this effect, lithium heparin concentration must be no more than 10 IU/mL. An alternative solution is to use specialized heparin preparations (calcium-balanced heparin, zinc heparin, blended zinc/lithium heparin) that effectively reduce the heparin binding effect to a clinically acceptable level.
Whatever the heparin formulation used, it is essential for accurate results that the correct volume of blood is sampled to achieve correct heparin concentration and that blood and anticoagulant are well mixed immediately after sampling.
Blood collected for serum estimation must be processed anaerobically. Samples should ideally be centrifuged at low temperature and the cap should not be removed prior to analysis. Hemolysis greater than 300 mg/dL hemoglobin causes clinically significant change in ionized calcium. Full recommendations for collection, transport and storage of specimens for ionized calcium are published [31].
Summary
The maintenance of plasma ionized calcium concentration within well-defined limits is essential for the many life-preserving physiological and cellular pathways that depend on ionized calcium. The action of two hormones, parathyroid hormone and calcitriol, is of major importance in this regard.
A range of clinical conditions – some very common – are associated with disturbance in calcium metabolism and resulting abnormality in ionized calcium concentration. If sufficiently severe, these changes in plasma ionized calcium concentration have profound adverse effect and may actually threaten survival.
Before the development of a reliable means of accurately measuring ionized calcium concentration in the mid-to-late 1980s, the only means of assessing calcium status was to measure total calcium concentration in plasma. Because this does not accurately reflect ionized calcium concentration in some clinical situations, it is a less satisfactory alternative.
For a number of mainly logistical reasons, it continues to be used, but measurement of ionized calcium rather than total calcium is widely considered mandatory for some patient groups, most notably the critically ill.
References+ View more
- Endres DB, Rude R. Mineral and bone metabolism. In: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (4th edition) Ed: Burtis C. Ashwood E. and Bruns D. 2006 Elsevier: Missouri
- Inzucchi S. Understanding hypercalcemia – its metabolic basis, signs and symptoms. Postgrad Med 2004; 115(4): 69-76
- Holick M, Krane S, Potts J. Calcium phosphorous and bone metabolism: calcium regulating hormones. In: Principles of Internal Medicine 1998. McGraw Hill: New York
- Brown E, Pollak M et al. Calcium ion sensing cell surface receptors. New Eng J Med 1995; 366: 575-80
- Robertson W, Marshall R Calcium measurement in serum and plasma. CRC reviews in Clin Lab Sci 1979; 11: 271-304
- Onifade K. Mohammed A, Peterson J et al. Ionized calcium: indications and advantages of its measurement. J Lab Med 2005; 29(4): 235-40
- McClean FC, Hastings AB. The state of calcium in the fluids of the human body. Conditions affecting ionization of calcium J Biol Chem 1935; 108: 285-322
- Parfitt A. Correction of plasma calcium measurements. Br Med J 1974; 1: 520
- Payne RB, Little AJ et al. Interpretation of serum calcium results in patients with abnormal serum proteins. Br Med J 1973; 4: 643-46
- Thode J, Juul-Jorgenson J et al. Comparison of serum total calcium, albumin corrected total calcium, and ionized calcium in 1213 patients with suspected calcium disorders. Scand J Clin Lab Invest 1989; 49: 217-23
- Orrel DH. Albumin as an aid to the interpretation of serum calcium. Clin Chim Acta 1971; 35: 483-89
- Conceicao S, Weightman D. Serum ionized calcium concentration: measurement versus calculation. Br Med J 1978; 1: 1103-05
- Zaloga G, Chernow B et al. Assessment of calcium homeostasis in the critically ill surgical patient. Annals of Surgery 1985; 202: 587-94
- Slomp J, van der Voort P et al. Albumin-adjusted calcium is not suitable for diagnosis of hyper – and hypocalcemia in the critically ill. Crit Care Med 2003; 31: 1393
- Dickerson R, Alexander K. Accuracy of methods to estimate ionized or ‘corrected’ serum calcium concentrations in critically ill multiple trauma patients receiving specialized nutrition support. J Parenteral and enteral nutrition 2004; 28: 133-41
- Byrnes M, Huynh K et al. A comparison of corrected serum calcium levels to ionized calcium levels among critically ill surgical patients. Amer J Surgery 2005; 189: 310-14
- Wang S, McDonnell E et al. pH effects on measurements of ionized calcium and ionized magnesium in blood. Arch Pathol Lab Med 2002; 126: 947-50
- Moore EW. Ionized calcium in normal serum, ultrafiltrates and whole blood determined by ion-selective electrodes. J Clin Invest 1970; 49: 318-14
- Larsson L Ohman S Serum ionized calcium and corrected total calcium in borderline hyperparathyroidism. Clin Chem 1978; 24: 1962-1965
- Goransson L, Skadberg O et al. Albumin-corrected or ionized calcium in renal failure. What to measure? Nephrol Dial Transplant 2005; 20: 2126-29
- Bushinsky DA, Monk R. Electrolyte quintet – Calcium. Lancet 1998; 352: 306-11
- Wong P, Carmecii C et al. Parathyroid crisis in a 20-year-old – an unusual cause of hypercalcaemic crisis. Postgrad. Med J 2001; 77: 468-70
- Takai E, Yano T et al. Tumour induced hypercalcemia and parathyroid hormone-related protein in lung carcinoma. Cancer 1996; 78: 1384-1387
- Kristensen B, Eljersten B et al. Survival in breast cancer patients after the first episode of hypercalcaemia. J Intern Med 1998; 244: 189-98
- Lamy O, Jenzer-Closuit A, Burckhard P. Hypercalcemia of malignancy: an underdiagnosed and undertreated disease. J Intern Med 2001; 250: 73-79
- Jacobs T, Bilezikian J. Clinical Review: Rare causes of hypercalcemia. J Clin Enodcrinol Metab 2005; 90: 6316-22
- Ralston S. Hypercalcaemia and hypocalcaemia In: Calcium metabolism. Medicine International 1993: 197-200
- Hastbacka J, Petilla V, Prevalence and predictive value of ionized hypocalcemia among critically ill patients. Acta Anasthesiol Scand 2003; 47: 1264-69
- Hsu S, Levine M. Perinatal calcium metabolism: physiology and pathophysiology. Seminars in Neonatology 2004; 9: 23-36
- Burnett R, Christiansen T et al. IFCC recommended reference method for the determination of the substance concentration of ionized calcium in undiluted serum plasma or whole blood. Clin Chem Lab Med 2000; 38: 1301-14
- NCCLS Ionized calcium determinations: Precollection variables, Specimen Choice, Collection and Handling: Approved guideline (2nd ed) NCCLS document C31-A2 2001 NCCLS Pennsylvania USA.
References
- Endres DB, Rude R. Mineral and bone metabolism. In: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (4th edition) Ed: Burtis C. Ashwood E. and Bruns D. 2006 Elsevier: Missouri
- Inzucchi S. Understanding hypercalcemia – its metabolic basis, signs and symptoms. Postgrad Med 2004; 115(4): 69-76
- Holick M, Krane S, Potts J. Calcium phosphorous and bone metabolism: calcium regulating hormones. In: Principles of Internal Medicine 1998. McGraw Hill: New York
- Brown E, Pollak M et al. Calcium ion sensing cell surface receptors. New Eng J Med 1995; 366: 575-80
- Robertson W, Marshall R Calcium measurement in serum and plasma. CRC reviews in Clin Lab Sci 1979; 11: 271-304
- Onifade K. Mohammed A, Peterson J et al. Ionized calcium: indications and advantages of its measurement. J Lab Med 2005; 29(4): 235-40
- McClean FC, Hastings AB. The state of calcium in the fluids of the human body. Conditions affecting ionization of calcium J Biol Chem 1935; 108: 285-322
- Parfitt A. Correction of plasma calcium measurements. Br Med J 1974; 1: 520
- Payne RB, Little AJ et al. Interpretation of serum calcium results in patients with abnormal serum proteins. Br Med J 1973; 4: 643-46
- Thode J, Juul-Jorgenson J et al. Comparison of serum total calcium, albumin corrected total calcium, and ionized calcium in 1213 patients with suspected calcium disorders. Scand J Clin Lab Invest 1989; 49: 217-23
- Orrel DH. Albumin as an aid to the interpretation of serum calcium. Clin Chim Acta 1971; 35: 483-89
- Conceicao S, Weightman D. Serum ionized calcium concentration: measurement versus calculation. Br Med J 1978; 1: 1103-05
- Zaloga G, Chernow B et al. Assessment of calcium homeostasis in the critically ill surgical patient. Annals of Surgery 1985; 202: 587-94
- Slomp J, van der Voort P et al. Albumin-adjusted calcium is not suitable for diagnosis of hyper – and hypocalcemia in the critically ill. Crit Care Med 2003; 31: 1393
- Dickerson R, Alexander K. Accuracy of methods to estimate ionized or ‘corrected’ serum calcium concentrations in critically ill multiple trauma patients receiving specialized nutrition support. J Parenteral and enteral nutrition 2004; 28: 133-41
- Byrnes M, Huynh K et al. A comparison of corrected serum calcium levels to ionized calcium levels among critically ill surgical patients. Amer J Surgery 2005; 189: 310-14
- Wang S, McDonnell E et al. pH effects on measurements of ionized calcium and ionized magnesium in blood. Arch Pathol Lab Med 2002; 126: 947-50
- Moore EW. Ionized calcium in normal serum, ultrafiltrates and whole blood determined by ion-selective electrodes. J Clin Invest 1970; 49: 318-14
- Larsson L Ohman S Serum ionized calcium and corrected total calcium in borderline hyperparathyroidism. Clin Chem 1978; 24: 1962-1965
- Goransson L, Skadberg O et al. Albumin-corrected or ionized calcium in renal failure. What to measure? Nephrol Dial Transplant 2005; 20: 2126-29
- Bushinsky DA, Monk R. Electrolyte quintet – Calcium. Lancet 1998; 352: 306-11
- Wong P, Carmecii C et al. Parathyroid crisis in a 20-year-old – an unusual cause of hypercalcaemic crisis. Postgrad. Med J 2001; 77: 468-70
- Takai E, Yano T et al. Tumour induced hypercalcemia and parathyroid hormone-related protein in lung carcinoma. Cancer 1996; 78: 1384-1387
- Kristensen B, Eljersten B et al. Survival in breast cancer patients after the first episode of hypercalcaemia. J Intern Med 1998; 244: 189-98
- Lamy O, Jenzer-Closuit A, Burckhard P. Hypercalcemia of malignancy: an underdiagnosed and undertreated disease. J Intern Med 2001; 250: 73-79
- Jacobs T, Bilezikian J. Clinical Review: Rare causes of hypercalcemia. J Clin Enodcrinol Metab 2005; 90: 6316-22
- Ralston S. Hypercalcaemia and hypocalcaemia In: Calcium metabolism. Medicine International 1993: 197-200
- Hastbacka J, Petilla V, Prevalence and predictive value of ionized hypocalcemia among critically ill patients. Acta Anasthesiol Scand 2003; 47: 1264-69
- Hsu S, Levine M. Perinatal calcium metabolism: physiology and pathophysiology. Seminars in Neonatology 2004; 9: 23-36
- Burnett R, Christiansen T et al. IFCC recommended reference method for the determination of the substance concentration of ionized calcium in undiluted serum plasma or whole blood. Clin Chem Lab Med 2000; 38: 1301-14
- NCCLS Ionized calcium determinations: Precollection variables, Specimen Choice, Collection and Handling: Approved guideline (2nd ed) NCCLS document C31-A2 2001 NCCLS Pennsylvania USA.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








