Printed from acutecaretesting.org
June 2000
Laboratory supervision of point-of-care blood gas
Introduction
The A.Z. Sint Jan AV, Brugge, Belgium, is a 916 bed tertiary care centre. Point-of-care (POC) blood gas testing, also offering K+ on selected analyzers, was gradually introduced in the early eighties to create the possibility of immediate clinical decision making in the critical care areas [1-3].
A total of six POC blood gas analyzers were installed in the intensive care (two analyzers), neonatal intensive care and emergency wards, in the operating rooms, and in the pneumology clinic. The STAT area of the central laboratory continued testing for other wards and functioned as a back-up.
As in many other institutions, routine maintenance and quality checks were performed daily by laboratory technicians. As no continuous monitoring of the instruments by the laboratory was possible, technical problems were often signalled by nurses too late, resulting in more serious problems and long down times.
Data transfer between POC analyzers and the Laboratory Information System (LIS) was very limited. Two instruments (ICU) were connected to the LIS by a coaxial cable, downloading test results with manually entered accession numbers and needing post hoc manually entry of patient demographics (35,000 samples/year).
Data entry of the other instruments was totally manual (16,000 samples/year).
Evaluation of the blood gas testing policy
As several analyzers needed replacement because of ageing, an internal audit on the justification and future of POC blood gas testing was conducted by the laboratory in close co-operation with the responsible clinicians during the fall of 1998. The conclusions of this audit were:
-
Continuation of POC blood gas testing on the wards as no other quick and reliable sample transport system was available. The installation of a pneumatic transport system was not considered an alternative because of the known pre-analytical interference by pressurised air [2,4].
-
Extension of the parameters to enhance clinical possibilities and to reduce the needed blood volume: blood gases, hemoglobin, K+ and glucose on all instruments; carboxyhemoglobin, Na+, Ca2+ and lactate on selected analyzers [2,5,6]. A dynamic parameter selection was mandatory because of legal obligations.
-
Analyzers had to be robust and easy to use (on-line help) for non-laboratory professionals to reduce operator errors, resulting in increased turnaround time [1]. The laboratory remains responsible for routine maintenance and quality control. A system for continuous monitoring of the instruments with possibility of remote control was considered essential to enhance availability of the instruments [2,3,7-9].
-
The training of the operators was assigned to the laboratory [3,7]. To prevent unauthorised actions or manipulation of the instruments by non-certified persons, instruments had to be equipped with mandatory login and customizable user profiles [2,7,9].
-
Data transfer to the LIS and hospital information system (HIS) had to be optimized to eliminate the tedious and error prone manual result entry [7,10].
-
All connections, for instrument monitoring and control as well as for data transfer, had to use by preference the existing Ethernet Local Area Network (LAN) [8].
-
Testing for the pneumology clinic was integrated into the STAT area of the laboratory because of the low sample number, the proximity (50 meters) to the laboratory, and the possibility of immediate electronic result reporting.
Introduction of an integrated instrument control and data management system
After extensive comparison of the solutions offered by different suppliers, we opted for buying the analyzers and the management system from the same manufacturer. The analyzer model met all of our prerequisites.
The connection of the analyzer over the LAN (Ethernet) with the management package is a standard feature as the analyzer is equipped with a network card and the software is based on the Windows™ 95 operating system. The management system was designed for Windows NT and comprises five modules.
The epicenter is the central data exchange module, which receives all data from the analyzers, sorts all data in the respective databases, and transmits results to the LIS/HIS. Data exchange between the analyzers and the management system was established with TCP/IP as a low level protocol, and uses a proprietary high level protocol. Data transmission to the LIS/HIS was configured with TCP/IP as low level protocol, and ASTM as high level protocol.
The analyzer control module permits continuous monitoring of the instrument status and review of the last patient and QC results. Activities which do not need physical intervention by the operator can be initiated: rinsing, cleaning, calibration, level sensing adjustment, lock/unlock and enter/exit standby. The supervisor has the possibility to send short messages from the management system PC to the analyzer display.
The patient data management module permits extensive review of test results. The analyzer data management module contains a full list of calibration results, QCs and system logs. The administrator module is used to configure the management system.
Prior to the introduction of the analyzers on the ward, all nurses and physicians (235) were familiarized with the working principles and measurement procedures by the medical laboratory technicians (MLT) responsible for POC testing or the supervising pathologist.
Daily maintenance and QCs are performed by one of the three POC MLTs. The MLTs responsible for the STAT section of the central laboratory received an extensive training in monitoring the analyzers using the management system and performing interventions in case of malfunction.
Fifteen volunteers of the nursing staff received additional training in routine replacement and trouble shooting procedures. A program for continuous education for all operators is being developed in collaboration with the nursing staff.
The measurement sequence starts with mandatory entry of a personal password. After presentation of the sample to the instrument and initiation of measurement, test parameters are selected using the touch screen. During sample measurement, mandatory identification of the patient and the requesting physician is done by barcode scanning.
Results, including technical remarks, are printed at the end of every measurement and transmitted by the instrument to the management system over the LAN. After inclusion of the results in its databases, the system sends the results in ASTM format to a dedicated PC, running ASTM-Net (Radiometer Medical A/S, Copenhagen, Denmark) which captures the data in a text file.
An in-house verification program written in Visual Basic 6.0 (Microsoft®, Redmont, WA, U.S.A.) eliminates result files from incorrectly identified samples and from interrupted measurements. After inclusion of a billing code, based on the day and time of measurement, the result file is transferred from the Windows-based LAN to our Wang®-based HIS by Light Speed (Lightspeed Software, Bakersfield, CA, USA).
A report is created by a dedicated background task from the text file (ASTM format) containing the identification of the patient, the requesting physician, and test results. Results are validated before final reporting on the LIS by the MLTs responsible for POC or by the supervising pathologist. This report is transmitted from the LIS to the HIS by the existing HL-7 transmission.
The whole data transfer process is summarized on Fig. 1. Data transfer, excluding result validation, takes less than three minutes.
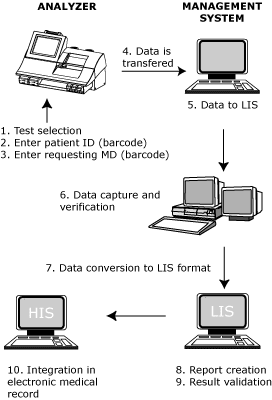 |
FIG 1: Data flow in a POC data management system. |
Results and conclusion
The whole process was progressively implemented over a period of nine months. After using the system for more than a year, the following results have been obtained and conclusions can be made:
- The audit and subsequent training of nurses and physicians, has greatly improved and intensified the contacts between the laboratory and the wards concerned.
- The continuous monitoring of the instruments is considered a major improvement, both by the nurses performing the analyses and by the MLTs.
- Daily maintenance of the instruments was shortened from three to two hours. Instrument failures due to empty reagent or full waste bottles and incorrect measurements due to calibration errors were nearly totally eliminated. Thanks to the possibility of continuous monitoring and remote control, minor problems can be solved from within the laboratory. The response to major errors was optimized as the remote instrument monitoring provides accurate information on the instrument status.
- The introduction of automated electronic data transfer to the LIS, has resulted in an important reduction of clerical workload. One full-time equivalent (FTE) was freed and was used for the introduction of new laboratory tests.
- The mandatory identification of patients by barcode scanning has also reduced data loss. This is best illustrated by a survey of the data collection on the emergency ward (Table I). When comparing the data collected during the 22 months prior to the chosen management system, with those collected during the 36 months following its introduction, a constant number of samples analyzed is observed. The number of samples with incorrect or missing identification was reduced from 39 per month before the implementation of the data management system with barcode identification to 13 per month after the introduction of the management system (Table I). Furthermore, the errors were often identified as barcode scanning errors and could mostly be manually corrected, leading to a drop in loss of data from 34 samples per month before to two after implementation.
- The addition of supplementary parameters has not led to an increased testing frequency. This is illustrated by the modest increase of samples on the IC ward. When comparing the average sample number per month registered during the year prior to the introduction of the management system, with the number collected during the year following the introduction, 2854 ± 232 (mean ± SD) versus 2982 ± 387, a small non-significant increase is observed. Moreover, this increase corresponds well to the estimated loss of about 100 samples during the audit.
- Automated, quick, and error free data transfer to the LIS has been introduced. The immediate transfer from the LIS to the HIS is much appreciated by clinicians.
|
|
TABLE I: Survey of the data collection in the emergency ward. Average sample number per month (± standard deviation) according to data collection
Although this system is an important step forward, efforts are being made to further enhance the possibilities of the system and to reduce imperfections. Recently, software has been developed to optimize patient identification.
After scanning the patient admission number, a query on the central patient database is performed for verification and translation of the admission code into a more meaningful patient name, sex, and birthday.
In the meantime, extensions of the existing modules and new functionalities of the chosen management system are being developed to expand monitoring of instrument and operator performance.
Acknowledgements
We would like to thank G. Waes and P. Maene for technical support and training of all operators. M. Van Cauwenberghe and F. Vandoorne are acknowledged for the assistance in the development of the in-house software. We also extend our gratitude to the laboratory staff, nurses, and physicians for their collaboration and continuous support.
Authors
Dirk Bernard, MD
Laboratorium voor Klinische Scheikunde
AZ Sint-Jan AVRuddershove
10B-8000 Brugge, Belgium
Daniel R. Vanhee
Laboratorium voor Klinische Scheikunde
AZ Sint-Jan AV
Brugge
Belgium
Victor H. Blaton
Laboratorium voor Klinische Scheikunde
AZ Sint-Jan AV
Brugge
Belgium
References+ View more
- Kilgore ML, Steindel SJ, Smith JA. Evaluating STAT testing options in an academic health center: therapeutic turnaround time and staff satisfaction. Clin Chem 1998; 44: 1597-1603.
- Harvey MA. Point-of-care laboratory testing in critical care. Am J Crit Care 1999; 8: 72-83.
- Kost GJ, Ehrmeyer SS, Chernow B, et al. The laboratory-clinical interface: Point-of-care testing. Chest 1999; 115: 1140-54.
- Astles JR, Lubarsky D, Loun B, et al. Pneumatic transport exacerbates interference of room air contamination in blood gas samples. Arch Pathol Lab Med 1996; 120: 642-47.
- Kost GJ. Guidelines for point-of-care testing. Improving patient outcomes. Am J Clin Pathol 1995; 104, Suppl 1: S111-27.
- Shirey TL. Critical care profiling for informed treatment of severely ill patients. Am J Clin Pathol 1995; 104, Suppl 1: S79-87.
- Check W. Connectivity at core of POC growing pains. CAP Critical Care information centre. http://www.cap.org/HTML/PUBLICATIONS/point/499cov.html
- Halpern NA, Brentjens T. Point of care testing informatics. Crit Care Clin 1999; 15: 577-91.
- Felder RA. The distributed laboratory: point-of-care services with core laboratory management. In: Point-of-care testing. Price CP, Hicks JM, eds. Washington DC: AACC Press. 1999: 99-118.
- Nichols JH. Practical management of point-of-care testing. Turn Around Times 1999; 2,2: 8.
References
- Kilgore ML, Steindel SJ, Smith JA. Evaluating STAT testing options in an academic health center: therapeutic turnaround time and staff satisfaction. Clin Chem 1998; 44: 1597-1603.
- Harvey MA. Point-of-care laboratory testing in critical care. Am J Crit Care 1999; 8: 72-83.
- Kost GJ, Ehrmeyer SS, Chernow B, et al. The laboratory-clinical interface: Point-of-care testing. Chest 1999; 115: 1140-54.
- Astles JR, Lubarsky D, Loun B, et al. Pneumatic transport exacerbates interference of room air contamination in blood gas samples. Arch Pathol Lab Med 1996; 120: 642-47.
- Kost GJ. Guidelines for point-of-care testing. Improving patient outcomes. Am J Clin Pathol 1995; 104, Suppl 1: S111-27.
- Shirey TL. Critical care profiling for informed treatment of severely ill patients. Am J Clin Pathol 1995; 104, Suppl 1: S79-87.
- Check W. Connectivity at core of POC growing pains. CAP Critical Care information centre. http://www.cap.org/HTML/PUBLICATIONS/point/499cov.html
- Halpern NA, Brentjens T. Point of care testing informatics. Crit Care Clin 1999; 15: 577-91.
- Felder RA. The distributed laboratory: point-of-care services with core laboratory management. In: Point-of-care testing. Price CP, Hicks JM, eds. Washington DC: AACC Press. 1999: 99-118.
- Nichols JH. Practical management of point-of-care testing. Turn Around Times 1999; 2,2: 8.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars









