Printed from acutecaretesting.org
December 2000
Myocardinal oxygen extraction after two months of adaptation to high altitude
INTRODUCTION
During exercise at sea level, the increase in myocardial O2 uptake is achieved primarily by an increase in coronary blood flow, which will be 4-5 fold during maximal exercise. An increase in relative myocardial O2 extraction contributes only slightly, the coronary sinus (cs) O2 saturation sO2(cs) decreasing from about 35 % at rest to 25 % during maximal exercise [1].
When a sea-level native is exposed acutely to hypoxia or simulated altitude in a low-pressure chamber, the increase in myocardial O2 uptake during exercise is covered by an increase in coronary blood flow, which may become about 35 % higher than during maximal exercise in normoxia. The relative myocardial oxygen extraction may increase slightly at rest, but not at all during heavy exercise, in which case sO2(cs) is the same as during normoxia [1]. Thus, the non-altitude-adapted man is fully dependent on the coronary flow reserve to tolerate acute exposure to hypoxia.
This is sufficient, without signs of anaerobic myocardial metabolism, to a level of arterial hypoxemia corresponding to 2,500-3,000 m.a.s.l. With hypoxemia corresponding to still higher altitude, myocardial V̇O2 falls short of that expected from the rate·pressure product, and in experiments where the blood pool of lactate has been labeled by 14C-lactate, a significant myocardial lactate production has been shown under such conditions [2]. To what extent the addition of anaerobic myocardial energy release is enough to maintain normal cardiac function, is not known.
The finding of the same rate·pressure products during maximal exercise under advanced hypoxemia as well as under normoxia would suggest that this is the case, but more subtle indices of cardiac function have not been applied in this situation.
Why the myocardium does not increase the relative O2 extraction during advanced hypoxemia is an enigma. However, there are data suggesting that physical training increases the relative myocardial O2 extraction (Kaijser, unpublished).
The objective of the present study was to reveal if a two-month stay at high altitude would adapt the myocardium to allow higher relative O2 extraction, which would permit a preserved myocardial aerobic metabolism during heavy exercise up to a higher altitude.
METHODS
Myocardial oxygen extraction and myocardial lactate metabolism were studied in six subjects immediately after the return from an 8-9-week sojourn at 5,200 m.a.s.l. by catheterizing an artery and the cs, utilizing coronary flow measurements by thermodilution. Measurements were taken at rest and during supine exercise at submaximal and maximal intensity breathing air, 12 % O2 (corresponding to 4,500 m.a.s.l.) and 6 % O2 (corresponding to about 7,000 m.a.s.l.). sO2, pO2, pCO2, lactate, and catecholamines were analyzed in arterial (a) and cs blood. Heart rate and intra-arterial pressure were followed continuously. Results were compared with those from non-acclimatized sea-level natives studied with the same exercise protocol breathing air and, acutely, 12 % O2 [1].
RESULTS
Breathing air, altitude-adapted subjects had, at rest, the same sO2(cs) as non-adapted subjects. However, during heavy exercise they had a greater myocardial O2 extraction, indicated by lower sO2(cs) (Fig. 1).
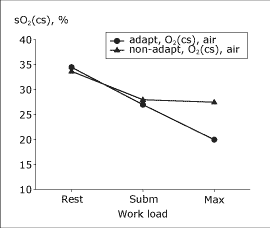 |
FIG. 1: Coronary sinus O2 saturation at rest, during submaximal and maximal exercise in non-adapted and altitude-adapted subjects breathing air at sea level. |
The difference between altitude-adapted and non-adapted subjects, when both groups were exposed to moderate hypoxemia breathing 12 % O2, was still greater. Furthermore, altitude-adapted subjects reached a higher maximal coronary blood flow and higher myocardial V̇O2. Breathing 6 % O2, which produced an sO2 during maximal exercise of about 45 %, sO2(cs) decreased further in the adapted subjects (Fig. 2).
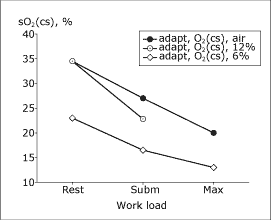 |
FIG. 2: Coronary sinus O2 saturation at rest and during exercise in altitude-adapted subjects breathing 12 % or 6 % O2. |
During maximal exercise breathing air, altitude-adapted subjects reach almost the same maximal arterial lactate concentration as non-adapted subjects. During air breathing as well as during 12 % O2 breathing, altitude-adapted subjects had a higher a-cs lactate difference, i.e., a higher lactate extraction, than non-adapted subjects in relation to the prevailing arterial lactate concentration, both during air breathing and during 12 % O2 breathing. Breathing 6 % O2, adapted subjects had at rest a normal myocardial lactate extraction, but during heavy exercise myocardial lactate net extraction was not significant, suggesting that substantial parts of the heart muscle had a partly anaerobic metabolism.
The altitude-adapted subjects had a greater maximal co-ronary sinus blood flow (left ventricular myocardial blood flow) and higher maximal myocardial O2 uptake than non-adapted subjects. To some extent this may have been the case already before the altitude sojourn and related to their higher maximal oxygen uptake.
DISCUSSION
The most striking finding in the altitude-adapted subjects was a greater relative extraction of O2 from the coronary vasculature, especially during heavy exercise, compared with non-adapted subjects. Furthermore, this difference was greater during exposure to hypoxia. As estimated from the relationship between myocardial O2 uptake and rate·pressure product, adapted subjects could maintain a normal myocardial O2 uptake at a lower capillary pO2.
This could have been the result of an increased mitochondrial density, and increased total activity of mitochondrial enzymes or of improved conditions for O2 diffusion from capillary to mitochondrion, possibly by increased capillary density. The altitude-adapted subjects also reached a significantly greater myocardial O2 uptake in addition to the factors mentioned above. This could have been the result of their larger maximal coronary blood flow.
If, to some extent, the larger maximal coronary blood flow was at hand already before the high-altitude sojourn, and if it was related to their higher maximal oxygen uptake (4.5 L/min compared to 3.8 L/min, which would indicate a larger stroke volume and larger left ventricular mass), is not possible to evaluate. However, the difference between the groups with regard to maximal coronary blood flow seems to be greater than the pre-existing difference in maximal oxygen uptake. The increased blood hemoglobin concentration after the altitude sojourn could also have contributed somewhat to the higher myocardial V̇O2.
At all levels of arterial oxygen tension, and at all levels of arterial lactate concentration, the myocardial lactate extraction was greater in altitude-adapted than in non-adapted subjects. This could have been a result of better oxygenation of the myocardium via the mechanisms discussed above. However, this difference was present also under conditions where the non-adapted subject showed no myocardial lactate release and therefore no sign of insufficient O2 supply.
Thus, there is a possibility that high-altitude adaptation includes a change in proportion of lactate dehydrogenase (LD) isoenzymes, with a relative increase in H compared with M subunits. If so, this would be in accordance with the adaptation to chronic myocardial ischemia in patients with coronary heart disease [3] as well as to the adaptation to chronic ischemia in skeletal musculature [4].
CONCLUSION
High-altitude adaptation in the form of an 8-9-week sojourn at 5,200 m.a.s.l. leads to an adaptation of the aerobic capacity of the myocardium, characterized by an increased ability to extract O2 from the coronary vascular bed. This may be the result of an increased capillarization, but also of an increased mitochondrial volume and activity of oxidative enzymes. It also seems as if myocardial lactate metabolism is altered, not only as a secondary result of increased aerobic capacity, but also caused by an altered proportion of LD-isoenzymes.
References+ View more
- Grubbström J, Berglund B, Kaijser L. Myocardial oxygen supply and lactate metabolism during marked arterial hypoxaemia. Acta Physiol Scand 1993; 149: 303-10.
- Kaijser L, Grubbström J, Berglund B. Myocardial lactate release during prolonged exercise under hypoxaemia. Acta Physiol Scand 1993; 149: 427-33.
- Lin L, Kaijser L, Liska J, et al. Increased expression of the lactate dehydrogenase M subunit in myocardial regions with decreased thallium uptake. Cardiovasc Res 1993; 27: 1300-05.
- Kaijser L, Sundberg CJ, Eiken O, et al. Muscle oxidative capacity and work performance after training under local leg ischemia. J Appl Physiol 1990; 69: 785-87.
References
- Grubbström J, Berglund B, Kaijser L. Myocardial oxygen supply and lactate metabolism during marked arterial hypoxaemia. Acta Physiol Scand 1993; 149: 303-10.
- Kaijser L, Grubbström J, Berglund B. Myocardial lactate release during prolonged exercise under hypoxaemia. Acta Physiol Scand 1993; 149: 427-33.
- Lin L, Kaijser L, Liska J, et al. Increased expression of the lactate dehydrogenase M subunit in myocardial regions with decreased thallium uptake. Cardiovasc Res 1993; 27: 1300-05.
- Kaijser L, Sundberg CJ, Eiken O, et al. Muscle oxidative capacity and work performance after training under local leg ischemia. J Appl Physiol 1990; 69: 785-87.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









