Printed from acutecaretesting.org
July 2005
Neonatal capillary blood sampling
INTRODUCTION
Capillary blood sampling (CBS) for laboratory testing is a standard procedure performed by various health professionals involved in the care of the neonate. In neonates it involves making an incision on the heel to obtain blood for sampling (heel lance) [1,2]. In general, laboratory specimens obtained from an artery or vein are regarded as the “gold standard” because they are perceived to reflect the body’s true values. However, sampling from arteries and veins is not always feasible and the risks associated with indwelling catheters such as thrombosis and infection limit the duration that they can be left in situ [2].
In addition, repeated venous sampling in hospitalized sick neonates may potentially limit the number of intravenous sites available for administration of total parenteral nutrition or medications.
Therefore, CBS remains the preferred method of obtaining small amounts of blood for laboratory analysis. As compared to venepuncture or arterial puncture, the advantages of heel lance (HL) include the ease and safety of the procedure, and results have shown to be comparable for most laboratory tests, with those from blood drawn from arterial catheters [3].
However, HL cannot be used to obtain large samples of volume or for specific testing such as a blood culture or coagulation profile.
HEEL LANCE AND PAIN
Preterm and term neonates undergoing HL cry and exhibit facial expression and body movements which are indicative of pain [4-11]. Several pharmacological and non-pharmacological interventions have been evaluated to reduce HL pain and the evidence is summarized below.
Pharmacological interventions
Acetaminophen [12] and topical anesthetics [13-17]
(lidocaine-prilocaine cream, amethocaine gel and 5 % lignocaine
ointment) are ineffective in decreasing HL pain.
Several trials have evaluated the efficacy of different sweetening agents. Administration of sucrose (dose range 0.012-0.12 g) approximately two minutes prior to HL when compared to water, pacifier or positioning/containment was effective in reducing pain [18]. Similarly, administration of glucose solution (10-50 % concentration) and breast-feeding was found to be effective [19.23].
Non-pharmacological interventions
Warming of the heel does not reduce pain or
facilitate blood collection by HL [24,25].
Using automated versus conventional lancets has been shown to reduce the duration of blood collection and indirectly the pain inflicted, degree of hemolysis of the blood sample obtained and bruising and inflammation of the heel [26-29]. As compared to HL, venepuncture is associated with less pain [30-33]. Comfort measures such as the use of pacifiers and rocking [34], non-nutritive sucking [35,36] and skin-to-skin contact (Kangaroo care) [37] are associated with reduction in HL pain.
Combinations of interventions
Several groups of investigators have shown that
administration of glucose and multisensory stimulation [38,39],
administration of sweet solutions followed by a pacifier [40] or
the use of sugar-coated pacifier [41] is effective in reducing HL
pain. Blass and Watt have shown that the combination of sucrose and
non-nutritive sucking was effective in reducing HL pain [42].
Gormally et al [43] showed that providing a sweet-tasting
solution and care-giving context (holding) may be a simple and
practical method of reducing pain in neonates subjected to painful
procedures.
In summary, the following recommendations can be made to reduce HL pain. Administration of a sweetening agent (sucrose or glucose on a pacifier) is a simple and effective way to reduce pain from single events. Automated lancet should be used for blood sampling and the heel should not be warmed prior to lancing. If feasible, parents should be encouraged to hold their baby during the procedure and mothers should be encouraged to breast- or bottle-feed during the procedure. Venepuncture is the preferred method of sampling in term neonates.
INDICATIONS FOR CBS
a) Any test where small volumes (< 1cc) of blood are required. These include:
- Hematological analysis (e.g. complete blood cell count)
- Biochemical analysis (e.g. electrolyte, glucose and bilirubin levels, therapeutic drug monitoring)
- Bedside accuchek/glucose estimation
- Metabolic screening (phenylketonuria and hypothyroidism).
b) Blood gas sampling [44] when:
- Arterial blood gas analysis is indicated but arterial access is not available
- Correlation with non-invasive monitor readings (e.g. transcutaneous values, end-tidal CO2)
- Assessment of initiation of therapeutic modalities (i.e. mechanical ventilation) and monitoring progression of the disease
HEEL-LANCE PROCEDURE
a) Site selectionIn neonates, the recommended site for sampling is on the plantar surface laterally beyond an imaginary line drawn posteriorly from between the 4th and 5th toes to the heel and medially from the middle of the great toe to the heel (FIGURE 1). This is based on the study by Blumenfeld et al [45] who evaluated the heels of 40 children weighing between 0.56 and 13.15 kg, 35 of whom were newborns at necropsy.
They measured the distance between the skin surface and the perichondrium of the calcaneus and in the smallest infant, the skin-perichondrium distance was documented to be 2.38 mm. In addition, they showed that the calcaneus rarely extended the recommended area of sampling. Thus, they recommended the maximum lancet depth of 2.4 mm to be used on the lateral or medial edges of the plantar surface to avoid damage to the calcaneus.
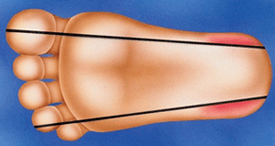
FIGURE 1: Appropriate puncture sites on the heel in neonates (darkened areas)
Based on the above guidelines only a limited area of the heel is available for sampling. With increasing survival of low-birth-weight infants, concerns have been raised regarding the effects of repeated HL in these infants with small feet. In addition, Fitzgerald et al [46] have shown that repeated HL leads to tissue injury and inflammation, making the heel more tender and hypersensitive to further lances.
Therefore to investigate whether it would be safe to extend the currently recommended area of sampling, Jain and Rutter [47] studied 80 infants weighing between 0.56 and 4.34 kg and with gestational age between 24 and 42 weeks. Using ultrasound they measured the shortest distance between the skin and the perichondrium of the calcaneus.
They showed that the shortest depth of perichondrium was in the center of the heel and ranged from 3 to 8 mm. In fact, in all but two of the infants the shortest skin-perichondrium distance was 4 mm or more, suggesting that a standard automated lancet that punctures to a depth of 2.4 mm can be safely used on any part of the plantar surface except the posterior aspect of the heel. Despite the findings of this study, the recommendations from the study by Blumenfeld et al are being used in clinical practice [45].
b) Equipment
The supplies needed to perform the procedure are listed in TABLE I. At our institution we are currently using an automated lancet to perform the procedure. They are available in two sizes, one for full-term neonate (incision depth of 1 mm and length of 2.5 mm) and one for preterm neonate (incision depth of 0.85 mm and length of 1.75 mm). Preheparinized capillary tubes are used for blood gas analysis.
| Non-sterile gloves | |
| Institution approved antiseptic swabs (alcohol/chlorhexidine swabs) | |
| Sterile gauze/cotton ball | |
| Sterile lancet (based on the institutional preference) | |
| Collection device depending on the type of test: | |
|
Microtainers |
|
| Capillary tubes (preheparinized), metal flea and magnet, caps for tubes at both ends | |
| Test strip | |
| Filter paper collection device | |
TABLE I: Equipment for heel-lance procedure
c) Technique/steps involved in the procedure
- The individual performing the procedure should wash his/her hands, wear gloves prior to the procedure and assemble the appropriate equipment.
- Warming the heel is NOT performed as it does not facilitate blood collection (volume and duration), does not reduce the infant’s response to pain or the number of repeat punctures.
- Pain-relieving interventions are administered based on institutional preference.
- The infant’s heel is held with a moderately firm grip. The forefinger is placed at the arch of the foot and the thumb below the puncture site at the ankle.
- After selection of the puncture site, the area is cleaned with an antiseptic swab and allowed to dry for maximum antiseptic action (approximately 30 seconds) and to prevent the mixture of blood and antiseptic solution.
- The base of the automated lancet is then placed flat against the heel, which is punctured using a quick controlled stroke.
- The first drop of blood is wiped off with a cotton ball or gauze and discarded as at it may be contaminated with skin cells, alcohol or excess tissue fluid, which may distort test results.
- The desired specimen is then obtained in an appropriate container. The heel is held in a dependent position and gentle pressure applied to facilitate blood flow. Excessive squeezing of the heel may cause hemolysis or contamination of the specimen from interstitial fluid leakage and bruising. In addition, the infant should be observed for any indication of pain such as behavioral (facial grimacing/crying/gross motor movements) and physiological responses (changes in heart rate, respiratory rate, blood pressure). If any of these are observed, the procedure should be discontinued to allow the baby to become calm or to provide additional comfort measures such as a pacifier, rocking or containment.
- Once the blood volume has been collected, the heel is cleansed of any residual blood. Gentle pressure with a cotton ball or gauze is then applied until the bleeding stops.
- Seal the specimen container and mix contents, if required.
- Blood gas testing: Once the capillary tube is filled with blood, place a finger over one end and cap the other end with commercially available covers. A metal flea is then placed in the capillary tube and the open end is capped. Holding the tube gently, the blood is mixed using a magnet back and forth along the outside of the tube.
d) Contraindications to HL [45,48]
- Presence of local edema or congestion as the accumulated fluid in the tissues will lead to contamination of the blood specimen.
- Presence of cyanosis or impaired perfusion as they will lead to distortion of the results or to inadequate sampling.
- Infection at the site as it can lead to local osteomyelitis or systemic infection.
- Puncturing previously traumatized skin can lead to increased pain and impaired blood coagulation.
e) Complications of HL include
- Infection including cellulitis, perichondritis, calcaneal osteomyelitis [49] and abscess are the major complications of this procedure. They can be prevented by using the recommended puncture areas, avoiding the tip of the heel and with the use of proper antiseptic techniques, using a sterile lancet for each puncture and by selecting a new site for each puncture. Neonates undergoing repeated heel pricks should be monitored for signs of infection such as redness, swelling and tenderness.
- Bruising can occur easily in the preterm neonates and is avoided by not using excessive or prolonged squeezing to obtain the specimen and correct positioning of the hand.
- Scarring and occurrence of calcified nodules in neonates subjected to repetitive punctures [50].
COMPARISON BETWEEN ARTERIAL AND CAPILLARY BLOOD LABORATORY RESULTS
Several studies have been published in the literature comparing blood gas results obtained from arterial and capillary sample with conflicting results. In 1990, Courtney et al [51] performed a study comparing postductal arterial and capillary blood gas measurements and reviewed the literature. The authors concluded that capillary blood gas measurements did not accurately predict arterial values in neonates.
However, Johnson et al [52] in 2000 compared laboratory results obtained from CBS using an automated incision device with those drawn from arterial catheters. There were no differences in pH, pCO2, lactate or sodium levels between the two methods of sampling. Levels for potassium, ionized calcium and lactate were noted to be significantly higher from the capillary sample but were within the acceptable CLIA performance criteria.
The levels for pO2 and glucose were markedly lower in the blood sample obtained via CBS. No differences were noted in the blood obtained from unwarmed versus warmed heels. In conditions where problems of oxygenation need to be delineated, arterial blood sampling should be obtained.
CONCLUSIONS
Heel lance remains the conventional method of blood sampling in neonates for screening tests (phenylketonuria and hypothyroidism) or obtaining blood for other laboratory investigations. Appropriate education and training of individuals performing the procedure is a necessary step in preventing complications. Warming of the heel has no effect on the outcome of sampling and therefore is an unnecessary step in the procedure. Pain-relieving interventions should be used to minimize the stress associated with HL.
Name of the institution where the work was performed
Department of Paediatrics, Mount Sinai
Hospital
Designation and Institutional Affiliation
[1] Staff Neonatologist, Mount Sinai Hospital
Assistant Professor, University of Toronto
[2] Scientist, Department of Pharmacy and Population Health
Sciences
The Hospital for Sick Children
Assistant Professor, University of Toronto
References+ View more
- Meehan RM. Heelsticks in neonates for capillary blood sampling. Neonatal Netw 1998;17:17-24.
- Bruck E. 1991. Procedures for the collection of diagnostic blood specimens by skin puncture, 3rd ed., Villanova, PA: National Committee for Clinical Laboratory Standards (NCCLS), document H4-A3.
- Yang K-C, Su B-H, Tsai F-J. The comparison between capillary blood sampling and arterial blood sampling in an NICU. Acta Paediatr Tw 2002; 43:124-26.
- Anand KJS, Hickley PR. Pain and its effect in the human neonate and fetus. N Engl J Med 1987;317:1321-29.
- Anand KJS, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress and analgesia in newborns and children. Pediatr Clin N Am 1989;36:795-818.
- Fitzgerald M, McIntosh M. Pain and analgesia in the newborn. Arch Dis Child 1989;64:441-43.
- Owens ME, Todt EH. Pain in infancy: neonatal reaction to heel lance. Pain 1984;20:77-86.
- Brown L. Physiologic responses to cutaneous pain in neonates. Neonatal Netw 1987;5:18-21.
- Izard CE. The maximally discriminative facial movement coding system (MAX). University of Delaware Instructional Resources Center: Newark DE, 1979.
- Grunau RVE, Craig KD. Pain expression in neonates: facial action and cry. Pain 1987;28:395-410.
- Johnston CC, Strada ME. Acute pain response in infants: a multidimensional description. Pain 1986;24:373-82.
- Shah V, Taddio A, Ohlsson A. Randomised controlled trial of paracetamol for heel prick pain in neonates. Arch Dis Child 1998;79:209-11.
- Ohlsson A, Taddio A, Jadad AR, Stevens BJ. Evidence-based decision making, systematic reviews and the Cochrane Collaboration: implications for neonatal analgesia. In: Anand KJS, Stevens BJ, McGrath PJ. Eds. Pain in Neonates, 2nd Revised and Enlarged Edition. Pain Research and Clinical Management, Vol 10, 2000, Elsevier Science BV: Amsterdam 2000.
- Larsson BA, Jylli L, Lagercrantz H, Ohlsson GL. Does a local anesthetic cream (EMLA) alleviate pain from heel-lancing in neonates. Acta Anaesthesiol Scand 1995;23:1028-31.
- Stevens B, Johnston C, Taddio A et al. Management of pain from heel lance with lidocaine-prilocaine (EMLA) cream: is it safe and efficacious in preterm infants? J Dev Behav Pediatr 1999;20:216-21.
- Jain A, Rutter N, Ratnayaka M. Topical amethocaine gel for pain relief of heel prick blood sampling: a randomised double-blind controlled trial. Arch Dis Child 2001;84:56-59.
- Rushforth JA, Griffiths G, Thorpe H, Levene MI. Can topical lignocaine reduce behavioural response to heel prick? Arch Dis Child 1995;72:49-51.
- Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Update Software: Oxford, 2003.
- Guala A, Giroletti G. Glucose as an analgesic in neonatology. A blind randomized controlled study. Pediatr Med Chir 1998;20:201-03.
- Gary L, Miller LW, Philipp BL, Blass EM. Breast feeding is analgesic in healthy newborns. Pediatrics 2002;109:590-93.
- Isik U, Ozek E, Bilgen H, Cebeci D. Comparison of oral glucose and sucrose solutions on pain response in neonates. J Pain 2000;1:275-78.
- Guala A, Pastore G, Liverani ME et al. Glucose or sucrose as an analgesic for newborns: a randomised controlled blind trial. Minerva Pediatr 2001;53:271-74.
- Skogsdal Y, Ericsson M, Schollin J. Analgesia in newborns given oral glucose. Acta Paediatr 1997;86:217-20.
- Barker DP, Willetts B, Cappendjik VC, Rutter N. Capillary blood sampling: should the heel be warmed? Arch Dis Child 1996;74:139-40.
- Janes M, Pinelli J, Landry S, Downey S, Paes B. Comparison of capillary blood sampling using an automated incision device with and without warming of the heel. J Perinatol 2002;22:154-58. 26.
- McIntosh N, van Veen L, Brameyer H. Alleviation of the pain of heel prick in preterm infants. Arch Dis Child 1994;70:177-181.
- Paes B, Janes M, Vegh P, LeDuca F, Andrew M. A comparative study of heel-stick devices for infant blood collection. Am J Dis Child 1993;147:346-48.
- Vertanen H, Fellman V, Brommels M, Viinikka L. An automatic incision device for obtaining blood samples from the heels of preterm infants causes less damage than a conventional manual lancet. Arch Dis Child 2001;84:53-55.
- Kellam B, Waller JL, McLaurin C et al. Tenderfoot preemie vs a manual lancet: A clinical evaluation. Neonatal Netw 2001;7:31-37.
- Shah V, Ohlsson A. Venepuncture versus heel lance for blood sampling in term neonates (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Update Software: Oxford, 2003.
- Shah VS, Taddio A, Bennett S, Speidel BD. Neonatal pain response to heel stick vs venepuncture for routine blood sampling. Arch Dis Child 1997;77:143-44.
- Larsson BA, Tannfeldt G, Lagercrantz H, Olsson GL. Venepuncture is more effective and less painful than heel lancing for blood tests in neonates. Pediatrics 1998;101:882-86.
- Eriksson M, Gradin M, Schollin J. Oral glucose and venepuncture reduce blood sampling pain in newborns. Early Hum Dev 1999;55:211-18.
- Campos RG. Rocking and pacifiers: two comforting interventions for heelstick pain. Res Nurs Health1 994;17:321-31.
- Corbo MG, Mansi G, Stagni A et al. Nonnutritive sucking during heelstick procedures decreases behavioral distress in the newborn infant. Biol Neonate 2000;77:162-67.
- Field T, Goldson E. Pacifying effects of nonnutritive sucking on term and preterm neonates during heelstick procedures. Pediatrics 1984;74:1012-15.
- Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics 2000;105. www.pediatrics.org/cgi/content/full/105/1/e14
- Bellieni CV, Buonocore G, Nenci A, Franci N, Cordelli DM, Bagnoli F. Sensorial stimulation: an effective analgesic tool for heel-prick in preterm infants: a prospective randomized trial. Biol Neoante 2001;80:15-18.
- Bellieni CV, Bagnoli F, Perrone S, et al. Effect of multisensory stimulation on analgesia in term neonates: a randomized controlled trial. Pediatr Res 2002;51:160-63.
- Akman I, Ozek E, Bilgen H, Ozdgoan T, Cebeci D. Sweet solutions and pacifiers for pain relief in newborn infants. J Pain 2002;3:199-202.
- Greenberg CS. A sugar-coated pacifier reduces procedural pain in newborns. Pediatr Nurs 2002;28:271-77.
- Blass EM, Watt LB. Suckling- and sucrose-induced analgesia in human newborns. Pain 1999;83:611-23.
- Gormally S, Barr RG, Werthiem L, Alkawaf R, Calinoiu N, Young SN. Contact and nutrient caregiving effects on newborn infants pain responses. Dev Med Child Neurol 2001;43:28-38
- AARC Clinical Practice Guideline. Capillary blood gas sampling for neonatal and pediatric patients. Respir Care 1994;39:1180-83.
- Blumenfeld T, Turi G, Blanc W. Recommended site and depth of newborn heel skin puncture based on anatomical measurements and histopathology. Lancet 1979;181:230-33.
- Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn and infants and its reversal with topical anesthesia. Pain 1989;39:31-36.
- Jain A, Rutter N. Ultrasound study of heel to calcaneum depth in neonates. Arch Dis Child 1999;80:243-45.
- Meites S. Skin-puncture and blood-collecting technique for infants: Update and problems. Clinical Chemistry 1988;34:1890-94.
- Lilien LD, Harris VJ, Ramamurthy RS, Pildes RS. Neonatal osteomyelitis of the calcaneus: Complication of heel puncture. J Paediatr 1976;88:478-80.
- Sell EJ, Hansen RC, Struck-Pierce S. Calcified nodules on the heel: A complication of neonatal intensive care. J Pediatr 1980;473-75.
- Courtney SE, Weber KR, Breakie LA, Malin SW, Bender CV et al. Capillary blood gases in the neonate. A reassessment and review of literature. Am J Dis Child 1990;144:168-72.
- Johnson KJ, Cress GA, Connolly NW, Burmeister LF, Widness JA. Neonatal laboratory blood sampling: Comparison of results from arterial catheters with those from an automated capillary device. Neonatal Netw 2000;19:27-34.
References
- Meehan RM. Heelsticks in neonates for capillary blood sampling. Neonatal Netw 1998;17:17-24.
- Bruck E. 1991. Procedures for the collection of diagnostic blood specimens by skin puncture, 3rd ed., Villanova, PA: National Committee for Clinical Laboratory Standards (NCCLS), document H4-A3.
- Yang K-C, Su B-H, Tsai F-J. The comparison between capillary blood sampling and arterial blood sampling in an NICU. Acta Paediatr Tw 2002; 43:124-26.
- Anand KJS, Hickley PR. Pain and its effect in the human neonate and fetus. N Engl J Med 1987;317:1321-29.
- Anand KJS, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress and analgesia in newborns and children. Pediatr Clin N Am 1989;36:795-818.
- Fitzgerald M, McIntosh M. Pain and analgesia in the newborn. Arch Dis Child 1989;64:441-43.
- Owens ME, Todt EH. Pain in infancy: neonatal reaction to heel lance. Pain 1984;20:77-86.
- Brown L. Physiologic responses to cutaneous pain in neonates. Neonatal Netw 1987;5:18-21.
- Izard CE. The maximally discriminative facial movement coding system (MAX). University of Delaware Instructional Resources Center: Newark DE, 1979.
- Grunau RVE, Craig KD. Pain expression in neonates: facial action and cry. Pain 1987;28:395-410.
- Johnston CC, Strada ME. Acute pain response in infants: a multidimensional description. Pain 1986;24:373-82.
- Shah V, Taddio A, Ohlsson A. Randomised controlled trial of paracetamol for heel prick pain in neonates. Arch Dis Child 1998;79:209-11.
- Ohlsson A, Taddio A, Jadad AR, Stevens BJ. Evidence-based decision making, systematic reviews and the Cochrane Collaboration: implications for neonatal analgesia. In: Anand KJS, Stevens BJ, McGrath PJ. Eds. Pain in Neonates, 2nd Revised and Enlarged Edition. Pain Research and Clinical Management, Vol 10, 2000, Elsevier Science BV: Amsterdam 2000.
- Larsson BA, Jylli L, Lagercrantz H, Ohlsson GL. Does a local anesthetic cream (EMLA) alleviate pain from heel-lancing in neonates. Acta Anaesthesiol Scand 1995;23:1028-31.
- Stevens B, Johnston C, Taddio A et al. Management of pain from heel lance with lidocaine-prilocaine (EMLA) cream: is it safe and efficacious in preterm infants? J Dev Behav Pediatr 1999;20:216-21.
- Jain A, Rutter N, Ratnayaka M. Topical amethocaine gel for pain relief of heel prick blood sampling: a randomised double-blind controlled trial. Arch Dis Child 2001;84:56-59.
- Rushforth JA, Griffiths G, Thorpe H, Levene MI. Can topical lignocaine reduce behavioural response to heel prick? Arch Dis Child 1995;72:49-51.
- Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Update Software: Oxford, 2003.
- Guala A, Giroletti G. Glucose as an analgesic in neonatology. A blind randomized controlled study. Pediatr Med Chir 1998;20:201-03.
- Gary L, Miller LW, Philipp BL, Blass EM. Breast feeding is analgesic in healthy newborns. Pediatrics 2002;109:590-93.
- Isik U, Ozek E, Bilgen H, Cebeci D. Comparison of oral glucose and sucrose solutions on pain response in neonates. J Pain 2000;1:275-78.
- Guala A, Pastore G, Liverani ME et al. Glucose or sucrose as an analgesic for newborns: a randomised controlled blind trial. Minerva Pediatr 2001;53:271-74.
- Skogsdal Y, Ericsson M, Schollin J. Analgesia in newborns given oral glucose. Acta Paediatr 1997;86:217-20.
- Barker DP, Willetts B, Cappendjik VC, Rutter N. Capillary blood sampling: should the heel be warmed? Arch Dis Child 1996;74:139-40.
- Janes M, Pinelli J, Landry S, Downey S, Paes B. Comparison of capillary blood sampling using an automated incision device with and without warming of the heel. J Perinatol 2002;22:154-58. 26.
- McIntosh N, van Veen L, Brameyer H. Alleviation of the pain of heel prick in preterm infants. Arch Dis Child 1994;70:177-181.
- Paes B, Janes M, Vegh P, LeDuca F, Andrew M. A comparative study of heel-stick devices for infant blood collection. Am J Dis Child 1993;147:346-48.
- Vertanen H, Fellman V, Brommels M, Viinikka L. An automatic incision device for obtaining blood samples from the heels of preterm infants causes less damage than a conventional manual lancet. Arch Dis Child 2001;84:53-55.
- Kellam B, Waller JL, McLaurin C et al. Tenderfoot preemie vs a manual lancet: A clinical evaluation. Neonatal Netw 2001;7:31-37.
- Shah V, Ohlsson A. Venepuncture versus heel lance for blood sampling in term neonates (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Update Software: Oxford, 2003.
- Shah VS, Taddio A, Bennett S, Speidel BD. Neonatal pain response to heel stick vs venepuncture for routine blood sampling. Arch Dis Child 1997;77:143-44.
- Larsson BA, Tannfeldt G, Lagercrantz H, Olsson GL. Venepuncture is more effective and less painful than heel lancing for blood tests in neonates. Pediatrics 1998;101:882-86.
- Eriksson M, Gradin M, Schollin J. Oral glucose and venepuncture reduce blood sampling pain in newborns. Early Hum Dev 1999;55:211-18.
- Campos RG. Rocking and pacifiers: two comforting interventions for heelstick pain. Res Nurs Health1 994;17:321-31.
- Corbo MG, Mansi G, Stagni A et al. Nonnutritive sucking during heelstick procedures decreases behavioral distress in the newborn infant. Biol Neonate 2000;77:162-67.
- Field T, Goldson E. Pacifying effects of nonnutritive sucking on term and preterm neonates during heelstick procedures. Pediatrics 1984;74:1012-15.
- Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics 2000;105. www.pediatrics.org/cgi/content/full/105/1/e14
- Bellieni CV, Buonocore G, Nenci A, Franci N, Cordelli DM, Bagnoli F. Sensorial stimulation: an effective analgesic tool for heel-prick in preterm infants: a prospective randomized trial. Biol Neoante 2001;80:15-18.
- Bellieni CV, Bagnoli F, Perrone S, et al. Effect of multisensory stimulation on analgesia in term neonates: a randomized controlled trial. Pediatr Res 2002;51:160-63.
- Akman I, Ozek E, Bilgen H, Ozdgoan T, Cebeci D. Sweet solutions and pacifiers for pain relief in newborn infants. J Pain 2002;3:199-202.
- Greenberg CS. A sugar-coated pacifier reduces procedural pain in newborns. Pediatr Nurs 2002;28:271-77.
- Blass EM, Watt LB. Suckling- and sucrose-induced analgesia in human newborns. Pain 1999;83:611-23.
- Gormally S, Barr RG, Werthiem L, Alkawaf R, Calinoiu N, Young SN. Contact and nutrient caregiving effects on newborn infants pain responses. Dev Med Child Neurol 2001;43:28-38
- AARC Clinical Practice Guideline. Capillary blood gas sampling for neonatal and pediatric patients. Respir Care 1994;39:1180-83.
- Blumenfeld T, Turi G, Blanc W. Recommended site and depth of newborn heel skin puncture based on anatomical measurements and histopathology. Lancet 1979;181:230-33.
- Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn and infants and its reversal with topical anesthesia. Pain 1989;39:31-36.
- Jain A, Rutter N. Ultrasound study of heel to calcaneum depth in neonates. Arch Dis Child 1999;80:243-45.
- Meites S. Skin-puncture and blood-collecting technique for infants: Update and problems. Clinical Chemistry 1988;34:1890-94.
- Lilien LD, Harris VJ, Ramamurthy RS, Pildes RS. Neonatal osteomyelitis of the calcaneus: Complication of heel puncture. J Paediatr 1976;88:478-80.
- Sell EJ, Hansen RC, Struck-Pierce S. Calcified nodules on the heel: A complication of neonatal intensive care. J Pediatr 1980;473-75.
- Courtney SE, Weber KR, Breakie LA, Malin SW, Bender CV et al. Capillary blood gases in the neonate. A reassessment and review of literature. Am J Dis Child 1990;144:168-72.
- Johnson KJ, Cress GA, Connolly NW, Burmeister LF, Widness JA. Neonatal laboratory blood sampling: Comparison of results from arterial catheters with those from an automated capillary device. Neonatal Netw 2000;19:27-34.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Minimizing pre-analytical errors in capillary blood sample collection and handling
Presented by Martha Lyon, PhD, Clinical Biochemist, Royal University Hospital, Saskatoon, Saskatchewan, Canada Watch the webinarRelated webinar
Cord blood gas analysis in obstetrical practice
Webinar presented by Jan Stener Jørgensen, MD PhD, Head of Obstetrics and Professor of Clinical Obstetrics, University of Southern Denmark Watch the webinarScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars










