Printed from acutecaretesting.org
October 2005
Patient safety: tested tools for error reduction
The Geisinger Health System is an integrated health delivery system serving a large rural geography in Central and Northeastern Pennsylvania.
Geisinger Medical Laboratories (GML) is the laboratory division of the Geisinger Health System. GML has a core laboratory at the flagship hospital in Danville and other laboratory operations in a community hospital in Wilkes-Barre and in more than 40 regional clinics.
GML employs about 425 people including 18 pathologists and doctoral directors. It has approximately 140 outreach clients and performs 4.7 million billable tests per year.
Laboratory errors can affect patient safety in a variety of ways. There is a risk of inappropriate treatment, delayed treatment or no treatment at all. There is also increased discomfort, anxiety and cost to the patient and to the laboratory when specimens have to be redrawn and retested.
At GML, the prevention of laboratory errors has been the motive for developing a series of programs and organizational structures over the last decade. Initially, medical technologists were promoted from testing positions to customer support roles in order to address preanalytical and postanalytical problems affecting the clinics that used our laboratory.
Subsequently, a formal Client Services Department was established with a call center.
Shortly after the report of the Institute of Medicine, “To Err is Human”, an error reduction program was announced. A Service Improvement Coordinator position was designed, a “morning report” focusing on problems and errors was established, electronic data collection tools were put in place and a monthly process error report covering all types of laboratory errors was developed.
A number of other activities were undertaken in order to raise the level of awareness and participation in error reduction within the laboratory (Figure I). This article highlights a number of these structures which we feel have proven useful in promoting error reduction and a culture of safety.
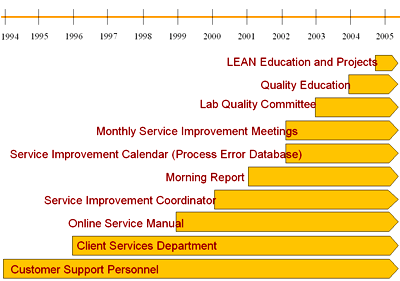
FIGURE I: Growth of service / Error reduction structures
CLIENT SERVICES DEPARTMENT
The better part of customer satisfaction is the avoidance of errors. Our Client Services Department professionalizes both error prevention and service recovery, setting the tone for the rest of our laboratory. Preventing errors in physician's offices and other client sites, which are not under our control, is a major focus of the Client Services Department.
Our department is made up of a team leader, two supervisory staff, five customer care representatives and 16 client service representatives. The customer care representatives are experienced medical technologists whose responsibilities include setup of new clients with supplies, specimen transport, information systems connectivity, requisition and reporting arrangements.
They provide technical support, customer service, continuing education and advocacy for their assigned clients. The client service representatives man the call center and resolve requests for add-on tests, supplies, courier pickups, laboratory results and phlebotomy.
Two workstations in the call center are devoted to the resolution of problems reported electronically from within the laboratory. The department employs a computer to track and trend external calls and monitor response times.
The Client Services Department maintains an online service manual and the department serves as a communication center for our entire client base, indispensable for managing any significant systemwide procedural change.
By attention to details at client sites and client education, this department greatly reduces preanalytical errors. Over 240 customized requisitions make it easy for customers to order correctly. Each error that occurs, e.g. improper collection, misidentified or lost specimens, is followed up with the responsible individuals at the client site by our staff.
Client Services also addresses postanalytical errors, e.g. misdirected reports, by maintaining up-to-date client demographics, preferred reporting sites and promptly fixing problems. By centralizing calls away from the testing areas, interruptions and distractions within the laboratory have been reduced and, presumably, analytical errors reduced.
Critical Value reporting has also been centralized, allowing us to document > 95 % of critical values reported within 30 minutes.
MORNING REPORT
This 8:00 a.m. meeting brings together the technical leadership of all areas of laboratory operation, including the laboratory sections, receiving and processing, Client Services, phlebotomy, courier, laboratory information systems, laboratory management and doctoral directors.
At the meeting significant errors, customer complaints and production impediments are brought forth from all parts of the lab for discussion, immediate resolution and the development of preventive actions. Client Services generally contributes several issues each day.
The issues are tracked in minutes published in the daily spreadsheet, which includes the immediate resolution, the preventive action and the individuals responsible for addressing the issue and following up. Issues remain on the daily minutes until completely resolved.
The database of issues can be searched by customer support staff to identify trends or the specific errors which have occurred related to a particular client (Figure II).
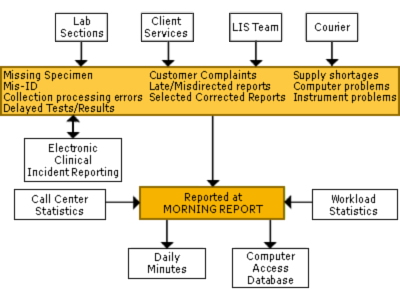
FIGURE II: Geisinger Medical Center Morning Report
Morning report has hugely increased the speed of resolution of problems because many angles to a problem are considered at once by the group. It has improved the thoroughness of follow-up and provided a focal activity within the laboratory, allowing all of lab management to know the major issues of the day.
Guests are invited to each meeting, which further disseminates the service culture.
COLLECTING ERRORS ELECTRONICALLY
Errors encountered in the Receiving and Processing section of the laboratory, e.g. improper collections, errors in storage or transit, identification errors, missing specimens are all transferred to Client Services for immediate resolution through a module of our laboratory information system.
This client services issues process (CSI) allows the problem to be passed to client services by entering basic information into the computer system and faxing the original requisition to Client Services.
CSIs are worked on promptly and the database is structured so that more than one person can work on the same problem simultaneously and problems can be passed from one shift to another smoothly. Major errors such as specimen misidentifications, and improper collection, misguided reports, etc. are brought to morning report for further discussion.
Non-actionable errors such as incorrect demographic information are excluded but continue to be tracked. The CSI process is also used by all the laboratory sections in multiple sites and in our two hospitals to report errors for centralized resolution (Figure III).
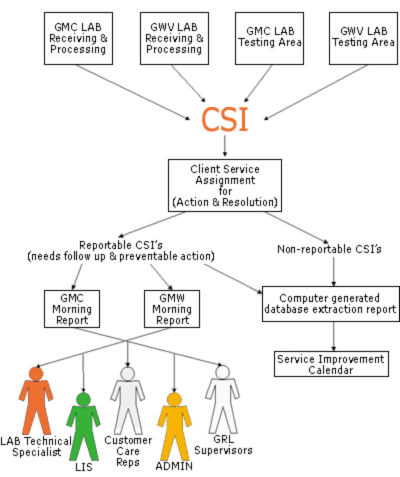
FIGURE III: Electric Customer Services Issues (CSIs)
SERVICE IMPROVEMENT CALENDAR
The Service Improvement Calendar is intended to be a comprehensive database of laboratory process errors. Twenty-seven categories of analytical, preanalytical and postanalytical errors are tracked and data is published every month both as absolute numbers of errors and as a percentage of billable tests.
Large parts of the data are derived from the laboratory information system’s reports on corrected results and deleted, credited or rejected tests. For each result modification, credit or deletion, a code is entered to allow tracking of the error or problem.
This data is manually reviewed prior to publication in the Service Improvement Calendar. Additional sources of statistics added to the Service Improvement Calendar include errors reported at morning report and errors reported electronically as hospital incident reports.
The assembly of this data allows us to identify the major error trends in the laboratory so they can be addressed. A longer-term goal is the desire to benchmark with other institutions regarding process errors.
OWNERSHIP OF ERRORS AND ERROR DATA
In our lab there was an initial reluctance to report errors; however, over time the benefit has become apparent to all involved. Ownership of the data and of the obligation to fix problems is a challenge which requires leadership from the top and also engagement of staff at all levels, including doctoral directors, supervisors, technical specialists, bench techs, phlebotomists, lab entry personnel.
Some of the tools that we have used to obtain ownership include a monthly Service Improvement meeting involving the lab technical leadership in which the Service Improvement Calendar is reviewed and where priorities are set for performance improvement projects. Successful performance improvement projects are reported by lab employees or supervisors at this meeting (see examples, Table I).
The discussion of error reduction has been a required agenda item for each laboratory section's regular meetings. Annual management goals for doctoral directors, supervisors and technical specialists have all included performance improvement projects or other error reduction efforts.
Several of these positions have monetary incentives as part of their compensation plan which have been linked to error reduction efforts. Performance improvement plans have been presented as posters which were visible for all in the laboratory.
|
Performance Improvement plans are based on service improvement opportunities & involve multidisciplinary efforts |
|
|
Table I: Performance Improvement Plans (PIP)
In some of the laboratory sections, including Receiving and Processing and Phlebotomy, the summary data for the section's performance is posted on the wall and tracked over time. Individuals can have access to their own data for errors, and in some instances error tracking is part of performance evaluation.
Over time, we have seen more and more engagement in error reduction and new enthusiasm has been built around small projects using LEAN principles, yielding positive results.
To promote ownership outside the laboratory, we provide regular reports to nursing management, the Emergency Department, the Neonatal ICU and the Performance Improvement Committee, regarding errors in identification, collection and clotted and hemolyzed specimens.
This information has led to increased cooperation with the laboratory to improve performance, increased involvement in education and the transfer of Phlebotomy services back to the laboratory.
POINT-OF-CARE OVERSIGHT
Without ongoing supervision and laboratory oversight, point-of-care testing (POCT) can deteriorate to a poor-quality operation with minimally trained personnel testing, poor-quality specimens tested on unmaintained equipment with outdated or improperly stored reagents.
In Geisinger hospitals, all POCT falls under the laboratory licenses. At Geisinger Medical Center, three full-time medical technologists are employed to supervise over 50 sites of POCT in clinics, patient units, ICUs, Emergency Departments and operating rooms.
Glucose testing is approximately 80 % of the volume, followed by blood gases, activated clotting tests and urinalyses. For most of these tests, the lab manages the flow of information into the laboratory information system and medical record through a special workstation.
Criteria have been established which trigger the review of unexpected results, which are then reviewed daily prior to posting.
The point-of-care staff are responsible for training POCT personnel, maintaining manuals, reviewing QC, assuring competency testing of personnel, data management and error tracking. Proficiency testing failures are addressed directly with staff and management.
Errors in patient specimen identification (usually entry errors by the users) are tracked for point-of-care glucose tests. Where 70 % of data entry is manual, nearly 3 % of our samples have misidentification errors. Where 70 % are entered with barcode scanners, errors are reduced to 1.2 %.
Using the barcode system, errors are around 1 in 2,000. Armed with this knowledge, we regularly provide nursing and the hospital Performance Improvement Committee with data on percent utilization of barcoded identification in POCT and the identification error rates.
These reports ultimately exert pressure on POCT personnel to follow a standard barcode wanding procedure.
SUMMARY
Patient safety, customer satisfaction and quality are aligned goals for laboratorians. At Geisinger, we have set up positions, meetings, committees and incentives to make improvements in our performance.
Major efforts and human investments have been made beyond traditional laboratory boundaries to improve pre- and postanalytical performance in client sites and other departments.
Simultaneously, we have invested heavily in electronic tools for error tracking, reporting and internal communication for the rapid resolution of errors. Point-of-care testing has been maintained under tight laboratory management.
The results of these efforts we believe have improved our ability to prevent errors at all phases of the laboratory testing cycle and to rapidly respond to and resolve errors.
A consistent leadership vision and steps taken over a number of years have led to an improved culture of safety and service.
References+ View more
- Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, D.C.: National Academy Press, 1999.
- Valenstein PN, Sirota RL. Identification errors in pathology and laboratory medicine. Clin Lab Med 2004; 24: 979-96.
- JCAHO 2005 National Patient Safety Goals from the website of the Joint Commission on Accreditation of Healthcare Organizations. www.jcaho.org
- Institute for Quality in Laboratory Medicine from the CDC website at www.iqlm.org
References
- Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, D.C.: National Academy Press, 1999.
- Valenstein PN, Sirota RL. Identification errors in pathology and laboratory medicine. Clin Lab Med 2004; 24: 979-96.
- JCAHO 2005 National Patient Safety Goals from the website of the Joint Commission on Accreditation of Healthcare Organizations. www.jcaho.org
- Institute for Quality in Laboratory Medicine from the CDC website at www.iqlm.org
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








