Printed from acutecaretesting.org
December 2009
Pediatric considerations in critical value assignment
A child knows you can't put a square peg in a round hole. Likewise, laboratory practices derived from adult clinical settings often do not fit the unique pediatric laboratory environment. In pediatrics, laboratory values must be interpreted in light of constant change.
The neonatal period to adolescence is marked by continuous linear growth, metabolic adaptations and neurologic development. The signal from critically important laboratory values must stand out from the noise of normal development. Failure to recognize these critical values has lifelong consequences.
In this report, critical value menus from chemistry laboratories in adult and pediatric institutions are compared and contrasted. Critical values unique to the pediatric environment will be identified and the rationale for their use will be discussed.
WHAT MAKES A VALUE "CRITICAL"
The concept of the critical value was originally defined by Lundberg in 1972 [1]. According to this definition, a critical value is one that represents imminent danger unless acted upon promptly.
In the pediatric sense, imminent danger certainly means loss of life but also refers to acute or latent development of physical or neurologic disability. Prompt intervention implies that the lab result is obtained proximal to the precipitating event and that intervention is clear-cut and immediate; a critical value should objectively beget treatment, not further diagnostic study.
When laboratories succumb to pressure to call back results that do not fit this definition, valuable technologist time is wasted and the importance of truly urgent situations is diluted.
THE CRITICAL VALUE LANDSCAPE – THEN AND NOW
There is a large body of literature devoted to critical values, but literature specific for pediatric settings is limited to a single report. In 1991, Kost et al. compared critical limits among 39 children's hospitals and contrasted these with a survey of predominantly adult hospitals [2].
In this report there was wide disparity between children's hospitals in both the quantity and numeric value of tests considered critical. The number of tests with critical limits ranged from 3 to 50. As one example of numeric disagreement within children's hospitals, critical glucose values ranged from 250 mg/dL to 1000 mg/dL.
There were, likewise, many numeric differences between pediatric and adult hospitals. For example, the median values for high critical limits for markers of renal function, urea nitrogen and creatinine were 50 % and 70 % lower in pediatric settings, respectively.
This wide range of tests and thresholds identified underscores the subjective nature of defining critical values.
Is the situation any different today? In 2008, the author re-examined the state of critical test menus from three adult hospitals, four pediatric institutions, and the suggested menu of critical tests found in the fourth edition of Tietz Textbook of Clinical Chemistry and Molecular Diagnosis.
The adult hospitals were all academic teaching centers ranging in size from 400 to 1000 beds and annual test volumes of 1.2 million to 10 million. The pediatric hospitals were, likewise, all university teaching hospitals ranging in size from 250 to 400 beds with annual test volumes from 400,000 to 700,000.
The number of tests with associated critical values remained highly variable. The number of tests with critical values ranged from 12 to 45 (Fig. 1).
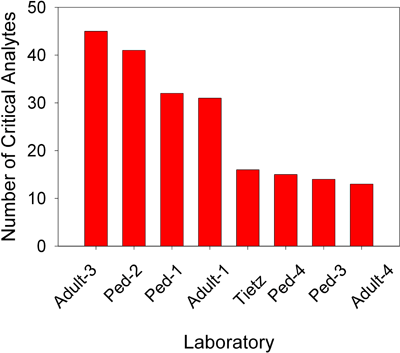
FIG. 1: Number of clinical chemistry analytes with associated critical values in four adult settings and four pediatric settings as compared to those suggested in Tietz Textbook of Clinical Chemistry (Table 56-3, Fourth edition).
Of the 62 analytes with critical values, 11 were unique to pediatric hospitals (Fig. 2).
These included (# of pediatric hospitals listing analyte as critical) total bilirubin (4), conjugated bilirubin (2), ammonia (2), albumin (1), AST (1), ALT (1), sweat chloride (1), iron (1), GGT (1), fibrinogen (1) and LDH (1).
These data clearly demonstrate that the definition of "critical" remains highly variable from site to site.
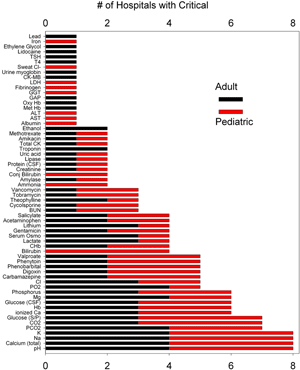
FIG. 2: Number of pediatric (red bar) and adult (black bar) settings with specific analytes designated with critical thresholds for physician call back.
ARE ALL CRITICAL VALUES CREATED EQUAL
The data published by Kost et al., the author's data and other reports [3, 4] suggest that Lundberg's original definition of the critical value has been stretched. One way around the critical/not critical conundrum is to reclassify such results into three tiers.
- The first tier, those life-and-death critical values that fit the Lundberg definition might be referred to as "must know now" results.
- The second tier, the "must know" results, would include data that are highly significant or diagnostic of a serious disorder, but do not necessarily dictate an immediate response.
- The third tier, the "courtesy" call, would include significantly abnormal results that might dictate a specific intervention but are context-dependent.
For example, calling back an ALT concentration of 300 U/L to a ward full of patients with liver disease will likely be met with indifference as virtually every patient may demonstrate such an abnormality, but the same call to an infectious-disease physician treating a patient with a potentially hepatotoxic drug might dictate a change in dose or medication.
Thus, the critical value decision involves not only the tests to associate with critical values but the actual value deemed critical.
In the next sections, specific examples of the qualitative and quantitative aspects of pediatric critical values will be examined.
CRITICAL VALUES UNIQUE TO PEDIATRICS – QUALITATIVE ANALYSIS
Figure 2 revealed 11 tests uniquely identified with critical values in pediatric hospitals. Three of these, total bilirubin, conjugated bilirubin and ammonia were identified by more than one hospital. Why are these analytes so critical in children?
a. Total Bilirubin
In the neonatal period, the turnover of red cells and production of bilirubin often exceed the conjugation capacity of the liver. The result is a transient unconjugated hyperbilirubinemia.
In general, concentrations of unconjugated bilirubin that exceed 15 mg/dL shortly after birth may be toxic to the neonatal central nervous system and may result in irreversible damage referred to as kernicterus.
The American Academy of Pediatrics has published recommendations for intervention based on total bilirubin concentrations as a function of age in hours [5]. Interventions to prevent kernicterus include phototherapy and exchange transfusion but these must be initiated promptly.
Thus, total bilirubin concentrations are ones that physicians must know now.
b. Conjugated bilirubin
While mild unconjugated hyperbilirubinemia occurs routinely in the neonatal period, elevated concentrations of conjugated bilirubin almost always indicate pathology. The differential diagnosis of neonatal conjugated hyperbilirubinemia is long, but one condition justifies prompt physician notification of this condition.
Biliary atresia is the congenital absence or obstruction of the common bile duct. The pathogenesis of this disorder is not known, but it does require surgery, known as the Kasai procedure, to establish bile flow from the liver to the small intestine.
Failure to recognize and surgically rectify biliary atresia within 1-2 months after birth results in irreversible liver damage for which the only resort is liver transplantation [6].
Elevated concentrations of conjugated bilirubin are often the first sign of biliary atresia but further radiographic and biochemical studies are required to establish the diagnosis.
Conjugated bilirubin concentrations, therefore, are ones that physicians must know, but not necessarily now, because biliary atresia is not imminently life-threatening and the course of action dictated by elevated concentrations is not clear-cut and immediate.
c. Ammonia
Ammonia is a neurotoxic byproduct of nitrogen metabolism. Detoxification normally occurs in the liver via the urea cycle. Hyperammonemia occurs in defects of the urea cycle or secondary to hepatocellular dysfunction. Regardless of the source, ammonia is toxic to the newborn brain.
Clear-cut and immediate interventions to decrease ammonia concentrations include an ammonia-scavenging cocktail (Ammonul®) or in extreme cases, hemodialysis. Elevated ammonia concentrations are ones that physicians must know now in order to institute appropriate therapy and prevent irreversible neurologic damage.
Ammonia concentrations can be elevated due to preanalytical variables, most commonly by delayed transport to the lab. The threshold for notification, therefore, should not be set too near the upper limit of the reference interval to avoid unnecessary physician notification.
The author's institution utilizes a threshold of 200 µmol/L as the trigger for notification.
CRITICAL VALUES UNIQUE TO PEDIATRICS – QUANTITATIVE ANALYSIS
Important decisions regarding the actual threshold for pediatric physician notification are certainly not limited to ammonia. A number of unique pediatric circumstances dictate quantitative triggers that differ from the adult settings. Two "must know now" situations will be examined in detail.
a. Potassium
Hyperkalemia is common in neonates. Physiologically, hyperkalemia results from slow maturation of both renal function and the sodium/potassium ATPase activity that maintains high intracellular potassium concentrations.
These findings are particularly common in low-birth-weight babies, but elevated potassium does not carry the same cardiac-conduction impact in neonates as it does in adults. Peaked T waves and prolonged QRS complex are not characteristic of mild-to-moderate hyperkalemia in infants. More commonly, blood collection artifacts lead to pseudohyperkalemia. Neonatal erythrocytes are more fragile than those from adults and neonatal blood is commonly obtained via capillary puncture.
These circumstances lead to in vitro hemolysis and high blood potassium concentrations. Hemolysis is often difficult to detect at the author's institution because basic electrolyte panels are often performed using whole blood to enhance turnaround time and conserve blood.
Residual whole blood is often not available to examine for hemolysis. Therefore, the high frequency of artifactual hyperkalemia and the relative resistance of infant hearts to mild hyperkalemia, permit a slightly higher critical trigger (as high as 7 or 8 mmol/L) in pediatric settings than in adult settings.
b. Total CO2
Small pediatric blood volumes commonly lead to falsely low blood carbon dioxide concentrations. The pCO2 in venous blood is >100 times the atmospheric pCO2.
Small blood volumes, therefore, lead to high surface-to-volume ratios and in succession, diffusion of CO2 from the collection tube, apparently low TCO2 concentrations, and often, an assumption of acidosis.
This situation may lead to further unnecessary investigation such as blood gas analysis or assessment for inborn metabolic disease and justifies a slightly lower critical limit for TCO2 in pediatric settings.
OTHER UNIQUE PEDIATRIC "CRITICAL" CIRCUMSTANCES
a. Newborn screening
Newborn screening is an exclusively pediatric enterprise. Positive screening tests must be followed by definitive confirmatory tests [7]. With the advent of expanded screening menus, efficient and appropriate mechanisms for notifying physicians of these results must be developed.
All of these conditions are potentially serious but all are not necessarily medical emergencies. Thus, they fall into the "must know" or "must know now" categories. A rationale for classification of some of these disorders is presented below.
Many inborn errors of metabolism uniformly result in life-threatening accumulation of toxic intermediates. Urea cycle disorders, phenylketonuria, and galactosemia are prime examples. Physicians must know about these disorders now to prevent dire consequences.
Another class of inborn errors poses the possibility of an acute life-threatening event. Fatty acid oxidation defects such as medium-chain acylCoA dehydrogenase (MCAD) deficiency may remain silent for extended periods, but after a prolonged fast, severe hypoglycemia may result in irreversible brain damage and death.
The severe potential of these disorders dictates that physicians know about these conditions now.
Another broad classification of conditions detectable in newborn screening programs are those that progress slowly and eventually lead to severe multi-organ dysfunction. For example, cystic fibrosis causes pancreatic insufficiency and chronic lung disease leading to respiratory failure in the third or fourth decade of life.
While acute life-threatening complications in the neonatal period are rare, evidence suggests that early intervention to clear the lungs and promote nutrient absorption delays the onset of severe symptoms. Physicians must, therefore, know about this condition in a timely manner, but not emergently.
Other disorders that may be similarly classified are congenital hypothyroidism and the various lysosomal storage diseases.
b. Drug monitoring/toxicology
There are few clear-cut differences between adult and pediatric settings in defining critically important drug monitoring or toxicology results. Critical thresholds for immunosuppressants, anticonvulsants and aminoglycosides are generally similar.
High-dose methotrexate therapy, however, is commonly utilized to treat acute childhood leukemia.
Each methotrexate concentration may be considered critical as oncologists must know now whether leucovorin treatment is indicated to avoid bone marrow toxicity [8]. Likewise, the detection of any exogenous toxin (e.g., drugs of abuse, acetaminophen, salicylate) may be considered critical as decisions regarding treatment and the provision for child protection must be considered promptly.
The author's institution has concluded that physicians must know about the detection of such substances now.
CONCLUSION
Classification of important laboratory results is more than an academic exercise. Prioritizing critical calls allows for the appropriate resources to be mobilized in conditions where life literally hangs in the balance (the "must know now" situations).
There is little alternative in these cases to an immediate discussion between the laboratory and the caregiver. In less urgent circumstances ("must know" and "courtesy" situations), data may be queued for delivery when time permits.
The phone may not always be the best way to relay such information. Taking advantage of alerting functions within electronic medical records, e-mail or other middleware solutions are possible modes of making sure important information is relayed without interrupting the normal workflow of caregivers.
As always, the threshold and urgency for notification should be jointly negotiated between clinicians and the laboratory and allow for evolution as diagnostic and therapeutic patterns change.
The pediatric environment is unique and sometimes requires a distinct set of analytes and numeric thresholds for physician notification. This environment absolutely deserves the extra scrutiny because in these instances, mistakes can last a lifetime.
References+ View more
- Lundberg GD. When to panic over abnormal values. MLO Med Lab Obs. 1972; 4: 47-54.
- Kost GJ. Critical limits for emergency clinician notification at United States children's hospitals. Pediatrics 1991; 88: 597-603.
- Lum G. Critical limits (alert values) for physician notification: universal or medical center specific limits? Ann Clin Lab Sci. 1998; 28: 261-71.
- Howanitz PJ, Steindel SJ, Heard NV. Laboratory critical values policies and procedures. Arch Pathol Lab Med. 2002; 126: 663-69.
- American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004; 114: 297-316.
- Erlichman J, Hohlweg K, Haber BA. Biliary atresia: how medical complications and therapies impact outcome. Expert Rev Gastroenterol Hepatol 2009; 3: 425-34.
- Dietzen DJ, Rinaldo P, Whitley RJ, Rhead WJ, Hannon WH, Garg UC, Lo SF, Bennett MJ. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: follow-up testing for metabolic disease identified by expanded newborn screening using tandem mass spectrometry. Clin Chem 2009; 55: 1615-26.
- Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem 1996; 42: 1322-29.
References
- Lundberg GD. When to panic over abnormal values. MLO Med Lab Obs. 1972; 4: 47-54.
- Kost GJ. Critical limits for emergency clinician notification at United States children's hospitals. Pediatrics 1991; 88: 597-603.
- Lum G. Critical limits (alert values) for physician notification: universal or medical center specific limits? Ann Clin Lab Sci. 1998; 28: 261-71.
- Howanitz PJ, Steindel SJ, Heard NV. Laboratory critical values policies and procedures. Arch Pathol Lab Med. 2002; 126: 663-69.
- American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004; 114: 297-316.
- Erlichman J, Hohlweg K, Haber BA. Biliary atresia: how medical complications and therapies impact outcome. Expert Rev Gastroenterol Hepatol 2009; 3: 425-34.
- Dietzen DJ, Rinaldo P, Whitley RJ, Rhead WJ, Hannon WH, Garg UC, Lo SF, Bennett MJ. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: follow-up testing for metabolic disease identified by expanded newborn screening using tandem mass spectrometry. Clin Chem 2009; 55: 1615-26.
- Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem 1996; 42: 1322-29.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









