Printed from acutecaretesting.org
October 2004
Permissive hypercapnea: Protecting the infant lung
History of chronic lung injury
The most common cause of neonatal death in the United States during the 1960s was hyaline membrane disease (HMD). Much was unknown about the disease at the time, but the next three decades would see an amazing growth in the understanding of the disease pathophysiology.
Surfactant deficiency and its role in the development of HMD was identified by Avery et al during the end of the 1950s [1]. Neonates suffering from HMD were recognized to have reduced lung compliance and functional residual capacity by Nelson et al soon thereafter [2].
Chu et al identified ventilation-perfusion mismatching and grunting as a mechanism for the infant to maintain recruitment and overcome ventilation-perfusion mismatching in hyaline membrane disease by the end of the 1960s [3].
Assisted ventilation of infants suffering from HMD soon followed. Initial strategies and technologies were crude and poorly met the infant’s needs. Initial experience showed that mechanical ventilation was able to change the course for some of these infants, but the survivors often suffered from chronic lung disease (CLD) [4-6].
Healthcare providers have been searching for methods to avoid the evolution of chronic lung disease associated with prematurity since the disease entity was first recognized and called bronchopulmonary dysplasia (BPD) by Northway et al in 1967 [7].
Despite advances in technology the development of lung injury secondary to the need for mechanical ventilation has continued to be a major problem for the preterm infant. BPD still affects 30-40 % of preterm infants needing mechanical ventilation [8].
BPD is a multifactorial condition related to the immaturity of the preterm infant’s lung and the injurious events that either accompany or follow the infant’s birth. The preterm infant’s lung and thorax are poorly equipped to handle the tidal volume breathing necessary once the infant is born.
The physical disadvantage of the infant’s thorax and the structural and biochemical immaturity of the lung lead to the need for mechanical support for adequate gas exchange.
Maintaining the proper inflation of the lung during the treatment of RDS is crucial in avoiding the atelectasis and trauma induced from the opening of collapsed alveoli. The importance of positive end expiratory pressure (PEEP) and its role in managing the infant with RDS has been known for quite some time [9].
It is now known that proper levels of PEEP are important in avoiding atelectotrauma and protecting the infant from developing BPD [9]. In 1974 Webb and Tierney reported that higher levels of PEEP were protective against the injury caused by higher inflation pressures in animals [10].
Others have since found the same findings in infants. In 1992 Van Mater et al reported their findings after examining the outcomes of infants from three different Harvard-associated nurseries. The authors found that infants exposed to higher levels of PEEP developed BPD less often [10].
Equally important in the evolution of BPD is the overdistention of alveoli that occurs when infants are ventilated at pressures that result in delivered tidal volumes above 4-6 mL/kg.
Volutrauma, as it has become known, is increasingly recognized as something to be avoided in an attempt to decrease the number of infants with BPD. Infants who experienced PaCO2 levels below the physiologic range have an increased risk of BPD, grade III/IV intraventricular hemorrhage, cystic periventricular leukomalacia and cerebral palsy [11,12].
Two articles published in 1989 and 1995 retrospectively evaluated the effects of hypocarbia on the outcomes of premature infants and found that it was associated with a greater risk of BPD [13,14]. Wanting to avoid overdistention and hypocarbia and avoiding them are often two separate issues.
The disease processes affecting a newborn are often moving targets and the clinical indicators lag behind the improving lung disease. Currently the best signs that an infant is receiving too much support are evidence of added pressure without continued increase in volume on the pressure-volume loop, and the laboratory evidence of hypocarbia (Fig. 1).
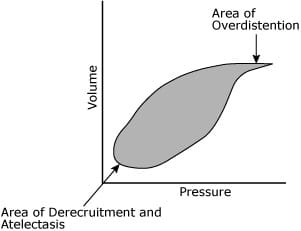
FIG 1.
The result of preterm birth, atelectotrauma, volutrauma and the exposure to oxygen is inflammation. Ogden et al in 1984 and Arnon et al in 1993 published evidence that mechanically ventilated infants who developed BPD had persistently elevated levels of neutrophils in bronchial lavage samples [15-17].
Other inflammatory mediators are also elevated in infants that develop BPD, including leukotrienes, platelet-activating factors and fibroblast-activating factors [18].
This inflammatory response starts the cascade that leads to parenchymal injury and remodeling that becomes BPD.
Clinical reasoning for permissive hypercapnea
During the past decade methods of management have changed to include more gentle ventilation, but only recently have results of randomized trials been published evaluating the approach of permissive hypercapnea.
The first experience with permissive hypercapnea as a strategy for managing patients needing assisted ventilation was in the treatment of adult respiratory distress syndrome during the late 1980s and early 1990s.
Studies in adults suffering from acute respiratory distress syndrome (ARDS) reported increased survival and fewer days on the ventilator in patients treated with pressure-limited ventilation to minimize potential barotrauma and allowing hypercapnea [19-21].
The first pediatric experience involved a patient suffering ARDS secondary to burn injuries treated successfully with a strategy involving pressure-limited ventilation and hypercapnea [22].
A subsequent retrospective study supported this approach to ventilation as potentially beneficial for pediatric patients suffering from ARDS secondary to burn injury [23].
The initial experience with permissive hypercapnea in the treatment of newborns came in the management of congenital diaphragmatic hernia. Centers began to move away from managing congenital diaphragmatic hernia with aggressive hyperventilation and alkalosis and employing a strategy involving lower peak inspiratory pressures or high frequency oscillatory ventilation and hypercapnea.
Infants treated in this way had improved outcomes and decreased mortality [24]. During the same period that authors were exploring the clinical application in children and infants, investigators started to examine the effects of elevated partial pressures of carbon dioxide (PaCO2) in models of lung injury.
Animal data suggests that hypercapnea is protective against multiple types of lung injury including ventilator-induced, endotoxin-induced and ischemia-reperfusion-induced lung injury [25-27].
There is no data to suggest harm from moderately elevated levels of PaCO2.
Randomized controlled trials
It was not until the late 1990s, however, that authors published results of randomized studies testing the hypothesis that gentle ventilation would yield improved results as compared to strategies pursuing normal carbon dioxide levels when treating preterm infants with respiratory distress.
Carlo et al published the results of a study that included 220 preterm infants ranging in birth weight from 501 to 1000 grams who were all mechanically ventilated at less than 12 hours of life. If the infants were between 751 and 1000 grams, they then had to require more than 30 % inspired oxygen and have received surfactant to be included in the study.
Infants were then randomized into two groups. The intervention group was managed with a strategy that included a target PaCO2 of > 52 mmHg, and a target of PaCO2 of < 48 mmHg was maintained in the control group [28].
These infants were part of a multicenter, randomized controlled trial of both the ventilatory strategies and early postnatal corticosteroids. The infants were managed in this regard for ten days. Definition of BPD in this group was oxygen requirement at 36 weeks postmenstrual age.
Mariani et al published the results of a study that included 49 newborn infants ranging in birth weight from 601 to 1250 grams. The infants all had RDS and were all mechanically ventilated at less than 24 hours of age.
The intervention group was managed to maintain PaCO2 values between 45 and 55 mmHg and maintain pH values > 7.2. The control group was managed with a strategy that included PaCO2 values from 35 to 45 mmHg and pH values > 7.25.
Infants were managed this way for 96 hours after which infants in the control group were managed simply to maintain pH values above the set criteria, allowing higher PaCO2 values in the control group [29].
Definition of BPD in this group was an oxygen requirement and abnormal x-ray on the 28th day of life.
Table I summarizes the findings of these trials.
Although some of the data imply that there may be both beneficial and harmful effects of permissive hypercapnea, none of the findings reached significance and no conclusions can be drawn from these data.
|
Relevative risk |
||
|
Outcome |
Mariani et al |
Carlo et al |
|
Death before discharge |
1.04 |
1.06 |
|
BPD at 28 days |
0.67 |
N/A |
|
CLD at 36 weeks |
1.05 |
N/A |
|
Death or CLD at 36 weeks* |
0.94 |
0.94 |
|
IVH all grades* |
0.82 |
0.82 |
|
IVH grades III/IV |
1.46 |
0.78 |
|
Periventricular leukomalacia |
1.04 |
1.06 |
|
Air leak |
0.52 |
N/A |
|
Pneumothorax |
N/A |
2.29 |
|
PIE |
N/A |
1.08 |
|
ROP grade 2 or above |
1.04 |
N/A |
TABLE I. Outcome data from Cochrane Database for RCTs comparing the strategy of normocapnia to permissive hypercapnea. *Indicates meta-analysis.
Current ventilation strategies
Although, to date, there is no evidence from randomized clinical trials (RCTs) that in human preterm infants a strategy including permissive hypercapnea leads to benefit, there is evidence in both humans and animals that pursuing normal PaCO2 levels with higher pressure and tidal volume is deleterious.
There is also evidence that several physiological states may actually be benefited by higher partial pressures of carbon dioxide. Because of these factors, the approach to ventilation has changed over the last decade to be more protective of the lung.
In our opinion, the goal for the premature neonate is to achieve a delivered tidal volume of 4-6 mL/kg delivered with a frequency that is appropriate for an infant, 40-60 breaths per minute.
The goal of this ventilation approach is no longer to have a ‘normal’ blood gas, but rather to achieve a PaCO2 range of 48-55 mmHg and a pH > 7.2. The inspired fraction of oxygen is aggressively decreased to maintain oxygen saturation values above 92 % and PaO2 levels > 60 mmHg.
Mechanical breaths are withdrawn from the infant as he/she contributes to the overall minute ventilation with spontaneous respiration; the ultimate goal being ventilation without assistance.
These goals are accomplished with a combination of newer ventilator technologies and the early use of a therapy that has been around for thirty years now: nasal continuous positive airway pressure (NCPAP).
The set pressure in time-cycled pressure-limited ventilation becomes a derivative of the infant’s disease state and response to therapies. Once the infant has reached sufficiently low support, extubation to NCPAP allows for spontaneous respiration while stabilizing the infant’s thorax and preventing atelectasis.
One of the most important additions to ventilator technology has been the addition of a pneumotachometer into the circuit itself. Not only does the information gathered give important insight into the disease process, but it also allows the care team to carefully deliver physiological tidal volumes.
A collection of the newer ventilators now incorporate the information from the pneumotachometer and a set tidal volume to deliver a consistent volume at the lowest possible pressure. This mode of ventilation has a variety of names depending upon the manufacturer, and the settings are slightly different among the different makers, but the concept is the same.
The patient receives a set volume every breath and the ventilator determines the pressure needed to deliver that breath based upon measurements made during prior delivered breaths. These breaths are delivered along with the patient’s own effort in a synchronized fashion.
The potential benefits are great to the neonate, allowing weaning from the support of the machine as the infant’s lung disease improves. This could be particularly beneficial to the newborn premature infant that has recently received surfactant.
A recent study published by Herrera et al demonstrated the effectiveness of this mode in a population of very-low-birth-weight infants.
In this study the short-term use of volume guarantee led to automatic weaning from mechanical support while maintaining ventilation and oxygenation [30].
Approaching the management of infants with lung disease in this manner is still evolving, and more randomized controlled trials need to be conducted to establish the benefit and safety of this approach.
Ideally, the management strategy for infants with lung disease would combine the above-mentioned approaches and technologies with available continuous monitoring of the infant’s PaCO2 by either end tidal carbon dioxide measurements or transcutaneous measurement of the tcpCO2.
In combination with continuous monitoring of the infant’s hemoglobin saturation via pulse oximetry this would allow the care team to be more responsive to the changing nature of the infant with lung disease while facilitating rapid adjustments in the infant’s support.
References+ View more
- Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child 1959; 97, 5, Part 1: 517-23.
- Nelson NM, Prod'Hom LS, Cherry RB, Lipsitz PJ, Smith CA. Pulmonary function in the newborn Infant. V. Trapped gas in the normal infant's lung. J Clin Invest 1963; 42: 1850-57.
- Chu J, Clements JA, Cotton EK, Klaus MH, Sweet AY, Tooley WH, et al. Neonatal pulmonary ischemia. I. Clinical and physiological studies. Pediatrics 1967; 40,4: Suppl:709-82.
- Downes JJ. Mechanical ventilation of the newborn. Anesthesiology 1971; 34,2: 116-18.
- Smith PC, Daily WJ, Fletcher G, Meyer HB, Taylor G. Mechanical ventilation of newborn infants. I. The effect of rate and pressure on arterial oxygenation of infants with respiratory distress syndrome. Pediatr Res 1969; 3,3: 244-54.
- Swyer PR. Results of artificial ventilation. Experience at the Hospital for Sick Children, Toronto. Biol Neonate 1970; 16,1: 148-54.
- Northway WH, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease: BPD. New England Journal of Medicine 1967; 276: 357-68.
- Davis J. Bronchopulmonary Dysplasia. In: Sinha S, Donn S, editors. Manual of neonatal respiratory care. Armonk: Futura Publishing Co.; 2000: 310-15.
- Dreyfuss D, Saumon G. Should the lung be rested or recruited? The Charybdis and Scylla of ventilator management. Am J Respir Crit Care Med 1994; 149,5: 1066-67.
- Van Marter LJ, Pagano M, Allred EN, Leviton A, Kuban KC. Rate of bronchopulmonary dysplasia as a function of neonatal intensive care practices. J Pediatr 1992; 120,6: 938-46.
- Wiswell TE, Graziani LJ, Kornhauser MS, Stanley C, Merton DA, McKee L, et al. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics 1996; 98,5: 918-24.
- Graziani LJ, Spitzer AR, Mitchell DG, et al. Mechanical ventilation in preterm infants: Neurosonographic and developmental studies. Pediatrics 1992; 90: 515-22.
- Garland JS, Buck RK, Allred EN, Leviton A. Hypocarbia before surfactant therapy appears to increase bronchopulmonary dysplasia risk in infants with respiratory distress syndrome. Arch Pediatr Adolesc Med 1995; 49,6: 617-22.
- Kraybill EN, Runyan DK, Bose CL, Khan JH. Risk factors for chronic lung disease in infants with birth weights of 751 to 1000 grams. J Pediatr 1989; 115,1: 115-20.
- Arnon S, Grigg J, Silverman M. Pulmonary inflammatory cells in ventilated preterm infants: effect of surfactant treatment. Arch Dis Child 1993; 69,1 Spec No: 44-48.
- Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis 1984; 130,5: 817-21.
- Ogden BE, Murphy S, Saunders GC, Johnson JD. Lung lavage of newborns with respiratory distress syndrome. Prolonged neutrophil influx is associated with bronchopulmonary dysplasia. Chest 1983; 83(5 Suppl): 31S-33S.
- Silverman M. Chronic lung disease of prematurity: are we too cautious with steroids? Eur J Pediatr 1994; 153,9 Suppl 2: S30-35.
- Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 1990; 16,6: 372-77.
- Network TARD. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301-08.
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338,6: 347-54.
- Reynolds EM, Ryan DP, Doody DP. Permissive hypercapnia and pressure-controlled ventilation as treatment of severe adult respiratory distress syndrome in a pediatric burn patient. Crit Care Med 1993; 21,6: 944-47.
- Sheridan RL, Kacmarek RM, McEttrick MM, Weber JM, Ryan CM, Doody DP et al. Permissive hypercapnia as a ventilatory strategy in burned children: effect on barotrauma, pneumonia, and mortality. J Trauma 1995; 39,5: 854-59.
- Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg 2002; 37,3: 357-66.
- Sinclair SE, Kregenow DA, Lamm WJ, Starr IR, Chi EY, Hlastala MP. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med 2002; 166,3: 403-08.
- Laffey JG, Tanaka M, Engelberts D, Luo X, Yuan S, Tanswell AK et al. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med 2000; 162,6: 2287-94.
- Laffey JG, Honan D, Hopkins N, Hyvelin JM, Boylan JF, McLoughlin P. Hypercapnic acidosis attenuates endotoxin-induced acute lung injury. Am J Respir Crit Care Med 2004; 169,1: 46-56.
- Carlo WA, Stark AR, Wright LL, Tyson JE, Papile LA, Shankaran S et al. Minimal ventilation to prevent bronchopulmonary dysplasia in extremely-low-birth-weight infants. J Pediatr 2002; 141,3: 370-74.
- Mariani G, Cifuentes J, Carlo WA. Randomized trial of permissive hypercapnia in preterm infants. Pediatrics 1999; 104,5 Pt 1: 1082-88.
- Herrera CM, Gerhardt T, Claure N, Everett R, Musante G, Thomas C et al. Effects of volume-guaranteed synchronized intermittent mandatory ventilation in preterm infants recovering from respiratory failure. Pediatrics 2002; 110,3: 529-33.
References
- Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child 1959; 97, 5, Part 1: 517-23.
- Nelson NM, Prod'Hom LS, Cherry RB, Lipsitz PJ, Smith CA. Pulmonary function in the newborn Infant. V. Trapped gas in the normal infant's lung. J Clin Invest 1963; 42: 1850-57.
- Chu J, Clements JA, Cotton EK, Klaus MH, Sweet AY, Tooley WH, et al. Neonatal pulmonary ischemia. I. Clinical and physiological studies. Pediatrics 1967; 40,4: Suppl:709-82.
- Downes JJ. Mechanical ventilation of the newborn. Anesthesiology 1971; 34,2: 116-18.
- Smith PC, Daily WJ, Fletcher G, Meyer HB, Taylor G. Mechanical ventilation of newborn infants. I. The effect of rate and pressure on arterial oxygenation of infants with respiratory distress syndrome. Pediatr Res 1969; 3,3: 244-54.
- Swyer PR. Results of artificial ventilation. Experience at the Hospital for Sick Children, Toronto. Biol Neonate 1970; 16,1: 148-54.
- Northway WH, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease: BPD. New England Journal of Medicine 1967; 276: 357-68.
- Davis J. Bronchopulmonary Dysplasia. In: Sinha S, Donn S, editors. Manual of neonatal respiratory care. Armonk: Futura Publishing Co.; 2000: 310-15.
- Dreyfuss D, Saumon G. Should the lung be rested or recruited? The Charybdis and Scylla of ventilator management. Am J Respir Crit Care Med 1994; 149,5: 1066-67.
- Van Marter LJ, Pagano M, Allred EN, Leviton A, Kuban KC. Rate of bronchopulmonary dysplasia as a function of neonatal intensive care practices. J Pediatr 1992; 120,6: 938-46.
- Wiswell TE, Graziani LJ, Kornhauser MS, Stanley C, Merton DA, McKee L, et al. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics 1996; 98,5: 918-24.
- Graziani LJ, Spitzer AR, Mitchell DG, et al. Mechanical ventilation in preterm infants: Neurosonographic and developmental studies. Pediatrics 1992; 90: 515-22.
- Garland JS, Buck RK, Allred EN, Leviton A. Hypocarbia before surfactant therapy appears to increase bronchopulmonary dysplasia risk in infants with respiratory distress syndrome. Arch Pediatr Adolesc Med 1995; 49,6: 617-22.
- Kraybill EN, Runyan DK, Bose CL, Khan JH. Risk factors for chronic lung disease in infants with birth weights of 751 to 1000 grams. J Pediatr 1989; 115,1: 115-20.
- Arnon S, Grigg J, Silverman M. Pulmonary inflammatory cells in ventilated preterm infants: effect of surfactant treatment. Arch Dis Child 1993; 69,1 Spec No: 44-48.
- Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis 1984; 130,5: 817-21.
- Ogden BE, Murphy S, Saunders GC, Johnson JD. Lung lavage of newborns with respiratory distress syndrome. Prolonged neutrophil influx is associated with bronchopulmonary dysplasia. Chest 1983; 83(5 Suppl): 31S-33S.
- Silverman M. Chronic lung disease of prematurity: are we too cautious with steroids? Eur J Pediatr 1994; 153,9 Suppl 2: S30-35.
- Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 1990; 16,6: 372-77.
- Network TARD. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301-08.
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338,6: 347-54.
- Reynolds EM, Ryan DP, Doody DP. Permissive hypercapnia and pressure-controlled ventilation as treatment of severe adult respiratory distress syndrome in a pediatric burn patient. Crit Care Med 1993; 21,6: 944-47.
- Sheridan RL, Kacmarek RM, McEttrick MM, Weber JM, Ryan CM, Doody DP et al. Permissive hypercapnia as a ventilatory strategy in burned children: effect on barotrauma, pneumonia, and mortality. J Trauma 1995; 39,5: 854-59.
- Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg 2002; 37,3: 357-66.
- Sinclair SE, Kregenow DA, Lamm WJ, Starr IR, Chi EY, Hlastala MP. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med 2002; 166,3: 403-08.
- Laffey JG, Tanaka M, Engelberts D, Luo X, Yuan S, Tanswell AK et al. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med 2000; 162,6: 2287-94.
- Laffey JG, Honan D, Hopkins N, Hyvelin JM, Boylan JF, McLoughlin P. Hypercapnic acidosis attenuates endotoxin-induced acute lung injury. Am J Respir Crit Care Med 2004; 169,1: 46-56.
- Carlo WA, Stark AR, Wright LL, Tyson JE, Papile LA, Shankaran S et al. Minimal ventilation to prevent bronchopulmonary dysplasia in extremely-low-birth-weight infants. J Pediatr 2002; 141,3: 370-74.
- Mariani G, Cifuentes J, Carlo WA. Randomized trial of permissive hypercapnia in preterm infants. Pediatrics 1999; 104,5 Pt 1: 1082-88.
- Herrera CM, Gerhardt T, Claure N, Everett R, Musante G, Thomas C et al. Effects of volume-guaranteed synchronized intermittent mandatory ventilation in preterm infants recovering from respiratory failure. Pediatrics 2002; 110,3: 529-33.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars









