Printed from acutecaretesting.org
July 2014
Point-of-care testing in the emergency department with emphasis on chest pain patients
The management at the emergency department of patients presenting with symptoms of cardiac disease such as chest pain and/or acute dyspnea accounts for a substantial proportion of emergency care resources.
Patients with symptoms of cardiac disease are high-risk patients and should be effectively identified by the emergency physician in order to initiate specific clinical actions.
Current recommendations identify the measurement of cardiac markers, i.e. cardiac troponins (cTn) and brain natriuretic peptides (BNP), as an important part of the diagnostic work-up, alongside other testing modalities such as ECG measurement and cardiac imaging.
Occasionally the measurement of D-dimer and blood gases may be indicated, e.g. in patients with suspected pulmonary embolism or patients suffering from cardiogenic shock.
The measurement at the point of care is a potential strategy for the reduction in turnaround time for cardiac marker testing in the emergency setting [1].
In this overview I have concentrated on the use of the cardiac biomarkers, BNP and cTn, in the diagnostic work-up in the emergency department.
However, the importance of cardiac troponins and natriuretic peptides for risk stratification is another very important aspect of these biomarkers, which is becoming increasingly important with the introduction of cardiac troponins with higher sensitivities.
In this respect many other biomarkers also seem to add to the outcome prediction of our patients with cardiac disease.
Thus, these aspects are outside the scope of this brief overview, which is a summary based on round-table discussions between experts in cardiology, emergency medicine and laboratory medicine and which were reported in two recent publications [1,3].
CARDIAC TROPONINS IN THE DIAGNOSIS OF ACUTE CORONARY SYNDROME
Increasing levels of cardiac troponins, either troponin I or troponin T, in blood signify damage to the myocardium.
The detection of circulating cardiac troponin is the most sensitive and specific biochemical marker of myocardial injury that is available to the physician in clinical settings, including the emergency department [4].
The early identification or ruling-out of the presence of an acute coronary syndrome relies on a combination of signs and symptoms, cardiac biomarker levels and findings in the ECG.
This is important, since signs and symptoms alone can be misleading, and since a substantial proportion of patients present with non-specific symptoms. Cardiac biomarkers can be elevated also in other conditions than acute coronary syndromes and findings in ECG are quite often unspecific [5,6].
Recent recommendations have noted that currently available cardiac troponin assays can contribute to rule-out protocols for myocardial infarction within 3 hours of arrival at the emergency department [7,8].
However, such protocols require the use of high-sensitive cardiac troponin assays currently only available at the clinical laboratory.
Contemporary studies using sensitive cardiac troponin assays show no added value of adding other markers of myocardial damages such as CKMB, myoglobin or Fatty-Acid Binding Protein [9,10].
The core principle underlying point-of-care measurement is the reduction in turnaround time without compromising the quality of information on which clinical decisions for patients are based [11].
A turnaround time of less than 1 hour should be achieved, and the availability of POCT could reduce the turnaround time to less than 30 minutes.
The 2011 guideline for management of non-ST-elevation acute coronary syndromes noted that a rapid (2-hour) rule-out protocol for acute coronary syndromes using POC testing of cardiac troponins, ECG and risk scoring was found to be safe [8,12].
Point-of-care systems should provide quantitative and not merely semiquantitative or qualitative measurement of cardiac troponin to support or rule out a diagnosis of acute myocardial infarction, given the importance of detecting changes in cardiac troponin levels for a correct diagnosis [13].
In addition, the sensitivity of the assay system should ideally not differ from that provided by central laboratory assays. The imprecision of the measurement at the 99th-percentile concentrations of cardiac troponins of healthy subjects has been highlighted as important in current management recommendations [14].
Specifically, imprecision of 10 % CV (coefficient of variation) or less at the 99th-percentile limit is desirable, and routine use of cardiac troponin assays with imprecision greater than 20 % at this limit is not recommended [15].
In order to be implemented successfully, a POCT for cardiac troponins should significantly reduce turnaround time without jeopardizing the diagnostic performance.
In particular, emergency physicians would tolerate neither a significant increase in false negative results which might lead to discharge of patients with a persistent risk of cardiac adverse events nor a substantial increase in “false positives” with the risk of unjustified and potentially hazardous therapeutic interventions.
The safety of an accelerated 90-minute protocol to exclude a diagnosis of acute myocardial infarction has been demonstrated in the emergency department setting, using serial multimarker POCTs (cardiac troponin, CKMB, myoglobin) [16].
All cases of acute myocardial infarction were diagnosed within 90 minutes, and admissions to the coronary care unit were decreased by 40 %. In addition, 90 % of patients with negative cardiac biomarkers and ECG findings were discharged, with only one returning with myocardial infarction within 1 month.
Another study focused on 817 consecutive emergency department admissions for suspected acute myocardial infarction [17].
The POCT strategy, which involved measurement of CKMB and cardiac troponin, provided a negative predictive value of 99.6 % for the diagnosis of myocardial infarction within 90 minutes, facilitating prompt and reliable rule-out of acute myocardial infarction.
The median turnaround time for the POCTs was 24 minutes, compared with 71 minutes for the central laboratory.
The Disposition Impacted by Serial Point of Care Markers in Acute Coronary Syndromes (DISPO-ACS) trial set out to test the hypothesis that POCT for cardiac troponin I would reduce length of stay, relative to central laboratory testing, in a large sample of 2000 patients evaluated for suspected acute coronary syndromes [18].
There was no overall change in length of stay. The authors concluded that reduced assay turnaround time per se was insufficient to influence length of stay, and that POCT must impact on other aspects of patient management in order to translate reduced assay turnaround time to more rapid discharge.
Reducing “brain-to-brain time” (the time between a physician ordering a test and when he/she interprets its results) was considered key to reducing overall length of stay.
A previous study from the USA involved 545 patients admitted to a cardiology unit with chest pain in roughly equal proportions immediately before or after the introduction of POCT for cardiac troponin I [19].
Charges to patients dropped by 25 % after the introduction of POCT, due to reduced costs associated with boarding, other departments, pharmacy, laboratories, and cardiac or non-cardiac procedures.
BRAIN NATRIURETIC PEPTIDES IN THE DIAGNOSIS OF HEART FAILURE
Dyspnea has been observed in about half of patients who received a primary diagnosis of heart failure in the ED, though it is well known to the ED physician that a number of other conditions present with dyspnea, including asthma, pulmonary edema, chronic obstructive pulmonary disease, interstitial lung disease and myocardial ischemia.
Rapid and accurate assessment of acute heart failure is therefore a priority when a patient presents with dyspnea in an emergency setting.
In recent years, the measurement of B-type natriuretic peptide (BNP) or NT-proBNP has become increasingly established for the management of patients presenting to an ED with dyspnea or other symptoms suggestive of heart failure in order to distinguish acute heart failure from these other conditions [20].
Point-of-care (POC) measurement of cardiac biomarkers has the potential for faster turnaround times and consequent increases in the speed of diagnosis and subsequent throughput of patients within the ED.
Data from randomized or observational clinical studies [21] [22] have generally demonstrated that the measurement of natriuretic peptides in the ED adds diagnostic information to other clinical measurements within a panel of biomarker and clinical measurements for the rule-in or -out of heart failure.
Importantly, the measurement of natriuretic peptides plus clinical judgment has been found to be superior to either alone. Major guidelines in the area support the measurement of natriuretic peptides most strongly where the clinical diagnosis of heart failure in patients presenting with acute dyspnea is uncertain.
A recent update of the main European guideline recommends measurement of natriuretic peptides in all patients with suspected heart failure [20].
Thus, rule-out may be possible with this measurement alone, without echocardiography, in patients with a normal ECG.
The measurement of NT-proBNP has been shown to result in significant cost savings relating to inpatient time, staff time, and usage of X-rays, echocardiography and treatments in a study of patients presenting to an ED with symptoms suggestive of heart failure (dyspnea or peripheral edema) [23].
Randomized studies have generally demonstrated reduced time to discharge following measurement versus no measurement of NT-proBNP with a tendency to reduce costs, or reduced time in hospital with use versus non-use of BNP testing at up to 1 year.
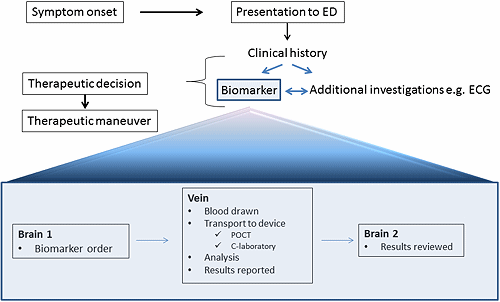
Fig.1:
POINT-OF-CARE TESTING FOR CARDIAC BIOMARKERS. WHY?
As outlined above point-of-care testing of cardiac troponins and natriuretic peptides does not offer any advantages over laboratory testing as to quality or sensitivity. Moreover, no studies have as yet shown that point-of-care testing saves lives.
Why then is point-of-care testing in the emergency setting so attractive and how can we take advantage of this technology? In the figure the work flow of a patient coming to the emergency department is outlined.
The key issues in relation to biomarker testing are three. Brain 1 is the time when a decision is made to request a biomarker test, Vein is the time it takes to draw blood, perform the analysis and report the results, and Brain 2 the time when the results are reviewed and action taken.
The time of Brain 1 to Vein is considerably shorter when point-of-care testing is performed as compared to laboratory testing because of transport time and other logistic issues.
The Vein to Brain 2 is the weak link. A common scenario is that Brain 1 to Vein takes 20 minutes and Vein to Brain 2 1-2 hours. Thus, in order to take full advantage of point-of-care testing this latter time must be shortened.
By shortening the time to a therapeutic decision and therapeutic maneuver we reduce the suffering for our patients and can quickly refer them to settings outside the emergency department, much to the relief of the overcrowded emergency departments. In the end this will also save money.
References+ View more
- Storrow AB, Lyon JA, Porter MW, Zhou C, Han JH, Lindsell CJ. A systematic review of emergency department point-of-care cadiac markers and efficiency measures. Point of Care 2009; 8: 121-25.
- Bingisser R, Cairns C, Christ M, Hausfater P, Lindahl B, Mair J, et al. Cardiac troponin: a critical review of the case for point-of-care testing in the ED. Am J Emerg Med 2012, 30:1639-1649.
- Bingisser R, Cairns CB, Christ M, Collinson P, Hausfater P, Lindahl B, et al. Measurement of natriuretic peptides at the point of care in the emergency and ambulatory setting: current status and future perspectives. Am Heart J 2013; 166,4: 614-21.
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008; 118,21: 2200-06.
- Steurer J, Held U, Schmid D, Ruckstuhl J, Bachmann LM. Clinical value of diagnostic instruments for ruling out acute coronary syndrome in patients with chest pain: a systematic review. Emerg Med J 2010; 27,12: 896-902.
- Body R, Carley S, Wibberley C, McDowell G, Ferguson J, Mackway-Jones K. The value of symptoms and signs in the emergent diagnosis of acute coronary syndromes. Resuscitation 2010; 81,3: 281-86.
- Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010; 122,17: 1756-76.
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011; 32,23: 2999-3054.
- Eggers KM, Oldgren J, Nordenskjold A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J 2004; 148,4: 574-81.
- Eggers KM, Venge P, Lindahl B. High-sensitive cardiac troponin T outperforms novel diagnostic biomarkers in patients with acute chest pain. Clin Chim Acta 2012; 413,13-14: 1135-40.
- Collinson PO. Cardiac biomarkers by point-of-care testing. In: Morrow DA, editor. Cardiovascular Biomarkers. Pathophysiology and Disease Management. Totowa, NJ: Humana Press, 2006: 559-74.
- Than M, Cullen L, Reid CM, Lim SH, Aldous S, Ardagh MW, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet 2011; 377,9771: 1077-84.
- Christenson RH, Phillips D. Sensitive and high sensitivity next generation cardiac troponin assays: more than just a name. Pathology 2011; 43,3: 213-19.
- Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010; 31,18: 2197-204.
- Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clin Chem 2010; 56,6: 941-43.
- Ng SM, Krishnaswamy P, Morissey R, Clopton P, Fitzgerald R, Maisel AS. Ninety-minute accelerated critical pathway for chest pain evaluation. Am J Cardiol 2001; 88,6: 611-17.
- McCord J, Nowak RM, McCullough PA, Foreback C, Borzak S, Tokarski G, et al. Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I. Circulation 2001; 104,13: 1483-88.
- Ryan RJ, Lindsell CJ, Hollander JE, O'Neil B, Jackson R, Schreiber D, et al. A multicenter randomized controlled trial comparing central laboratory and point-of-care cardiac marker testing strategies: the Disposition Impacted by Serial Point of Care Markers in Acute Coronary Syndromes (DISPO-ACS) trial. Ann Emerg Med 2009; 53,3: 321-28.
- Apple FS, Chung AY, Kogut ME, Bubany S, Murakami MM. Decreased patient charges following implementation of point-of-care cardiac troponin monitoring in acute coronary syndrome patients in a community hospital cardiology unit. Clin Chim Acta 2006; 370,1-2: 191-95.
- Thygesen K, Mair J, Mueller C, Huber K, Weber M, Plebani M, et al. Recommendations for the use of natriuretic peptides in acute cardiac care: A position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur Heart J 2012; 33,16: 2001-06.
- Meisel SR, Januzzi JL, Medvedovski M, Sharist M, Shochat M, Ashkar J, et al. Pre-admission NT-proBNP improves diagnostic yield and risk stratification - the NT-proBNP for EValuation of dyspnoeic patients in the Emergency Room and hospital (BNP4EVER) study. Eur Heart J Acute Cardiovasc Care 2012; 1,2: 99-108.
- Rehman SU, Martinez-Rumayor A, Mueller T, Januzzi JL, Jr. Independent and incremental prognostic value of multimarker testing in acute dyspnea: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Clin Chim Acta 2008; 392,1-2: 41-45.
- Mueller C, Laule-Kilian K, Schindler C, Klima T, Frana B, Rodriguez D, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med 2006; 166,10: 1081-87.
References
- Storrow AB, Lyon JA, Porter MW, Zhou C, Han JH, Lindsell CJ. A systematic review of emergency department point-of-care cadiac markers and efficiency measures. Point of Care 2009; 8: 121-25.
- Bingisser R, Cairns C, Christ M, Hausfater P, Lindahl B, Mair J, et al. Cardiac troponin: a critical review of the case for point-of-care testing in the ED. Am J Emerg Med 2012, 30:1639-1649.
- Bingisser R, Cairns CB, Christ M, Collinson P, Hausfater P, Lindahl B, et al. Measurement of natriuretic peptides at the point of care in the emergency and ambulatory setting: current status and future perspectives. Am Heart J 2013; 166,4: 614-21.
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008; 118,21: 2200-06.
- Steurer J, Held U, Schmid D, Ruckstuhl J, Bachmann LM. Clinical value of diagnostic instruments for ruling out acute coronary syndrome in patients with chest pain: a systematic review. Emerg Med J 2010; 27,12: 896-902.
- Body R, Carley S, Wibberley C, McDowell G, Ferguson J, Mackway-Jones K. The value of symptoms and signs in the emergent diagnosis of acute coronary syndromes. Resuscitation 2010; 81,3: 281-86.
- Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010; 122,17: 1756-76.
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011; 32,23: 2999-3054.
- Eggers KM, Oldgren J, Nordenskjold A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J 2004; 148,4: 574-81.
- Eggers KM, Venge P, Lindahl B. High-sensitive cardiac troponin T outperforms novel diagnostic biomarkers in patients with acute chest pain. Clin Chim Acta 2012; 413,13-14: 1135-40.
- Collinson PO. Cardiac biomarkers by point-of-care testing. In: Morrow DA, editor. Cardiovascular Biomarkers. Pathophysiology and Disease Management. Totowa, NJ: Humana Press, 2006: 559-74.
- Than M, Cullen L, Reid CM, Lim SH, Aldous S, Ardagh MW, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet 2011; 377,9771: 1077-84.
- Christenson RH, Phillips D. Sensitive and high sensitivity next generation cardiac troponin assays: more than just a name. Pathology 2011; 43,3: 213-19.
- Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010; 31,18: 2197-204.
- Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clin Chem 2010; 56,6: 941-43.
- Ng SM, Krishnaswamy P, Morissey R, Clopton P, Fitzgerald R, Maisel AS. Ninety-minute accelerated critical pathway for chest pain evaluation. Am J Cardiol 2001; 88,6: 611-17.
- McCord J, Nowak RM, McCullough PA, Foreback C, Borzak S, Tokarski G, et al. Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I. Circulation 2001; 104,13: 1483-88.
- Ryan RJ, Lindsell CJ, Hollander JE, O'Neil B, Jackson R, Schreiber D, et al. A multicenter randomized controlled trial comparing central laboratory and point-of-care cardiac marker testing strategies: the Disposition Impacted by Serial Point of Care Markers in Acute Coronary Syndromes (DISPO-ACS) trial. Ann Emerg Med 2009; 53,3: 321-28.
- Apple FS, Chung AY, Kogut ME, Bubany S, Murakami MM. Decreased patient charges following implementation of point-of-care cardiac troponin monitoring in acute coronary syndrome patients in a community hospital cardiology unit. Clin Chim Acta 2006; 370,1-2: 191-95.
- Thygesen K, Mair J, Mueller C, Huber K, Weber M, Plebani M, et al. Recommendations for the use of natriuretic peptides in acute cardiac care: A position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur Heart J 2012; 33,16: 2001-06.
- Meisel SR, Januzzi JL, Medvedovski M, Sharist M, Shochat M, Ashkar J, et al. Pre-admission NT-proBNP improves diagnostic yield and risk stratification - the NT-proBNP for EValuation of dyspnoeic patients in the Emergency Room and hospital (BNP4EVER) study. Eur Heart J Acute Cardiovasc Care 2012; 1,2: 99-108.
- Rehman SU, Martinez-Rumayor A, Mueller T, Januzzi JL, Jr. Independent and incremental prognostic value of multimarker testing in acute dyspnea: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Clin Chim Acta 2008; 392,1-2: 41-45.
- Mueller C, Laule-Kilian K, Schindler C, Klima T, Frana B, Rodriguez D, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med 2006; 166,10: 1081-87.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Register for the related webinar
The Benefit of Using a D-dimer Assay with a High Clinical Specificity
Presented by Karin Strandberg, MD, PhD, Assoc. Prof.
Join the webinarAcute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars