Printed from acutecaretesting.org
December 1999
Point-of-care testing for blood gases and electrolytes
|
TABLE I: Potential benefits of point-of-care testing for blood gases and electrolytes
For the clinical pathologist, it is essential to be familiar with the evidence to support new technologies. We should also be creative in developing novel applications for the instruments designed by the diagnostics industry.
From a clinical perspective, point-of-care testing may yield a number of benefits, not all of which are strictly medical in nature (Table I). Various studies have reported on the impact of POCT for blood gases and electrolytes in different hospital settings.
For example, Kendall et al [2] reported a randomized controlled trial of POCT in an emergency department compared to testing performed in the clinical laboratory. POCT for arterial blood gases resulted in medical decisions being made 21 minutes earlier than when testing was performed in the central laboratory.
However, there was no improvement in the time patients spent in the emergency department, length of stay, admission rate, or mortality.
Parvin et al [3] prospectively evaluated use of a POCT device for measuring Na, K, Cl, glucose, and BUN in an emergency department setting and found no difference in emergency department length of stay compared to testing in the central laboratory.
However, other studies have suggested that length of stay in an emergency department can be reduced using POCT technologies [4]. Kost has pointed out that POCT using a limited menu without a care pathway or treatment algorithm may produce little benefit unless the overall integration of the technology into the medical care event has been addressed [5].
The value of POCT in neonatal/pediatric settings is more established. VanNewKirk et al reported a retrospective study of utilization, iatrogenic blood loss, and transfusion rates in a neonatal intensive care unit following implementation of a POCT device for blood gases [6].
Blood loss per admission was reduced from 24.1 to 6.7 mL, utilization of testing decreased from 80 to 66 tests per admission, and the rate of transfusions decreased from 8 to 6 (not statistically significant).
The benefits of POCT for reducing iatrogenic blood loss in the NICU setting have been confirmed by other investigators [7,8].
Although the above comments concerning the clinical utility of POCT for blood gases and electrolytes do not reflect the entire body of literature on this subject, they serve to demonstrate that clinical outcomes data remain limited, are at best neutral, and provide no clear direction as to the desirability of POCT from the perspective of the clinician.
This contrasts to the situation for capillary blood glucose testing in the management of diabetes mellitus, where significant improvement in patient outcomes has been demonstrated in various settings [9].
Lacking a consensus in the medical literature on the benefit to patients of POCT for blood gases and electrolytes, the major issue remains one of cost and the impact of these technologies on hospital operations.
Unfortunately, the literature on the financial aspects of POCT for blood gases and electrolytes is confusing and in some cases contradictory. The literature concerning the cost of POCT is most developed for bedside glucose testing [10].
However, Winkelman et al have challenged the premise of POCT on fiscal and operational grounds [11] for both capillary blood glucose testing and acute care applications.
A number of sources have reported improved turnaround time and decreased unit and overall cost associated with POCT for blood gases and electrolytes. For example, Bailey et al [1] reported an institutional savings of USD 392,336 with a reduction in unit cost from 15.33 per panel to 8.03 following institution-wide implementation of POCT blood gas analysis.
Other studies have shown contradictory results. Kost has concluded that “there is no adequate generalization.
Cost-effectiveness analysis, consideration of marginal costs of competing alternatives, and other means of economic assessment must be individualized to the institution and setting” [5].
We agree. Lacking clear outcomes data and with no consensus concerning issues of cost effectiveness, the decision of whether to implement POCT versus using a central laboratory will depend on a number of features unique to the individual hospital or care unit within the institution.
Of necessity, the approach must be in part intuitive and may require trial and error to arrive at a satisfactory solution.
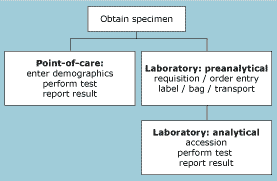
FIG. 1: Model of activities in point-of-care testing and in central laboratory testing
At the Massachusetts General Hospital, we have experimented with different models for providing blood gas and electrolyte, testing including neonatal and acute care satellite laboratories, central laboratory testing supported by a pneumatic tube system, and recently pilot efforts to implement POCT in the neonatal intensive care unit.
These efforts have been evolutionary, not revolutionary. Currently, we are planning to evaluate the POCT option. A preliminary cost analysis suggests point-of-care testing may lower unit panel cost from USD 7.02 in the laboratory to USD 5.08 at the point of care [12].
Additional benefits may include savings in laboratory space, reduced turnaround time, a reduction in pneumatic tube traffic, and reduced iatrogenic blood loss. The model on which the cost analysis is based is relatively simple, as shown in Fig. 1.
Performance of a laboratory-based test includes the labor and consumable costs incurred in the laboratory and those associated with nursing activities, including requisitions, specimen labeling, and preparing the specimen for pneumatic tube transport.
Some functions are common to both the laboratory-based test and those performed at the point of care (draw blood, result reporting).
Point-of-care testing requires entry of patient and operator data, and performance of the test. In the laboratory, the specimen is received, accessioned, the test performed, and the result reported.
Our concept is that the labor required by the nurse to perform the point-of-care test is roughly equivalent to that of obtaining and preparing a sample for the clinical laboratory.
The nursing labor therefore cancels out of the equation and the analysis simplifies to the consumable cost of the point-of-care test versus the unit cost (labor and consumables) of the central laboratory (Table II).
If nurse labor is the same for both POCT and
laboratory
|
|
|
Comparative cost
|
Comparative cost
|
TABLE II: Cost analysis of point-of-care testing versus the central laboratory: a hypothetical model for blood gas/electrolyte testing.
Whether our model is accurate in actual practice remains to be seen. First, we have assumed no change in test utilization. Furthermore, the analysis requires that POCT essentially replaces the clinical laboratory.
Partial implementation of POCT results in a completely different scenario. In this case, central laboratory testing is reduced, but not eliminated.
The tests removed from the
central laboratory therefore reflect marginal costs, and the
financial impact on the laboratory would be minimal.
In conclusion it is evident from the above discussion that
many issues concerning the clinical utility and cost-effectiveness
of POCT technologies for blood gases and electrolytes remain
unresolved.
Improvements in analytical technologies, data management systems, and expansion of the test menu will introduce new challenges and opportunities.
Laboratory professionals should consider POCT technologies as a possible tool to improve patient care while maintaining a healthy skepticism until more reliable data concerning cost and effectiveness are available.
References+ View more
- Bailey TM, Topham TM, Wantz S, Grant M, Cox C, Jones D, Zerbe T, Spears T. Laboratory process improvement through point-of-care testing. Jt Comm J Qual Improv 1997; 23: 7.
- Kendall J, Reeves B, Clancy M. Point-of-care testing: randomized controlled trial of clinical outcome. BMJ 1998; 316.
- Parvin CA, Lo SF, Deuser SM, Weaver LG, Lewis LM, Scott MG. Impact of point-of-care testing on patients’ length of stay in a large emergency department. Clin Chem 1996; 42,5: 711-17.
- Sands VM, Auerbach PS, Birnbaum J, Green M. Evaluation of a portable clinical blood analyzer in the emergency department. Acad Emerg Med 1995; 2: 172-78.
- Kost, GJ. Point-of-care testing in intensive care. Principles and Practice of Intensive Care Monitoring; 74; 1267-96.
- VanNewkirk LE, Bhutani VK, Husson MA, Warhol MJ. Impact of reducing blood sample size on the incidence of transfusion in a neonatal ICU. Laboratory Medicine 1998; 29: 5.
- Despotis GJ, Santoro SA, Spitznagel E, et al. Prospective evaluation and clinical utility of on-site monitoring of coagulation in patients undergoing cardiac operation. J Thorac Cardiovasc Surg 1994; 107: 271.
- Despotis GJ, Joist JH, Goodnough LT. Monitoring of hemostasis in cardiac surgical patients: impact of point-of-care testing on blood loss and transfusion outcomes. Clin Chem 1997; 43,9: 1684-96.
- Lewandrowski L, Lewandrowski K. Bedside capillary glucose testing in the management of patients with diabetes mellitus: clinical utility and relationship to patient outcomes. In: Hicks J, ed. Point-of-Care Testing. American Association for Clinical Chemistry Press. In press.
- Lewandrowski E, Laposata M, Eschenbach K, et al. Utilization and cost analysis of bedside capillary glucose testing in a large teaching hospital: implications for the management of point-of-care testing. Amer J Med 1994; 97: 222-30.
- Winkelman JW, Wybenga DR. Quantification of medical and operational factors determining central versus satellite laboratory testing of blood gases. Am J Clin Pathol 1994; 102: 7-10.
- Letts G, Palmer-Toy D, Lewandrowski K. Process improvement and reorganization in an adult acute care laboratory: a 4-year experience in a large teaching institution. In: Proceedings of the 17th International Symposium of the Electrolyte/Blood Gas Division American Association for Clinical Chemistry, Nice, France, 1998.
References
- Bailey TM, Topham TM, Wantz S, Grant M, Cox C, Jones D, Zerbe T, Spears T. Laboratory process improvement through point-of-care testing. Jt Comm J Qual Improv 1997; 23: 7.
- Kendall J, Reeves B, Clancy M. Point-of-care testing: randomized controlled trial of clinical outcome. BMJ 1998; 316.
- Parvin CA, Lo SF, Deuser SM, Weaver LG, Lewis LM, Scott MG. Impact of point-of-care testing on patients’ length of stay in a large emergency department. Clin Chem 1996; 42,5: 711-17.
- Sands VM, Auerbach PS, Birnbaum J, Green M. Evaluation of a portable clinical blood analyzer in the emergency department. Acad Emerg Med 1995; 2: 172-78.
- Kost, GJ. Point-of-care testing in intensive care. Principles and Practice of Intensive Care Monitoring; 74; 1267-96.
- VanNewkirk LE, Bhutani VK, Husson MA, Warhol MJ. Impact of reducing blood sample size on the incidence of transfusion in a neonatal ICU. Laboratory Medicine 1998; 29: 5.
- Despotis GJ, Santoro SA, Spitznagel E, et al. Prospective evaluation and clinical utility of on-site monitoring of coagulation in patients undergoing cardiac operation. J Thorac Cardiovasc Surg 1994; 107: 271.
- Despotis GJ, Joist JH, Goodnough LT. Monitoring of hemostasis in cardiac surgical patients: impact of point-of-care testing on blood loss and transfusion outcomes. Clin Chem 1997; 43,9: 1684-96.
- Lewandrowski L, Lewandrowski K. Bedside capillary glucose testing in the management of patients with diabetes mellitus: clinical utility and relationship to patient outcomes. In: Hicks J, ed. Point-of-Care Testing. American Association for Clinical Chemistry Press. In press.
- Lewandrowski E, Laposata M, Eschenbach K, et al. Utilization and cost analysis of bedside capillary glucose testing in a large teaching hospital: implications for the management of point-of-care testing. Amer J Med 1994; 97: 222-30.
- Winkelman JW, Wybenga DR. Quantification of medical and operational factors determining central versus satellite laboratory testing of blood gases. Am J Clin Pathol 1994; 102: 7-10.
- Letts G, Palmer-Toy D, Lewandrowski K. Process improvement and reorganization in an adult acute care laboratory: a 4-year experience in a large teaching institution. In: Proceedings of the 17th International Symposium of the Electrolyte/Blood Gas Division American Association for Clinical Chemistry, Nice, France, 1998.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar










