Printed from acutecaretesting.org
October 2005
Preanalytical issues related to blood sample mixing
INTRODUCTION
Homogeneity of sample achieved by adequate mixing of the blood sample is the key to the quality of analytical results.
Immediate mixing of the sample upon blood collection insures that the anticoagulant in the specimen container has fully dissolved with the blood, thus maintaining not only the effective anticoagulant-to-blood ratio but also providing a specimen free from any blood clots.
The process of mixing should be such as not to result in the alteration of the integrity of the sample, such as hemolysis, and thus affect the quality of results.
HEMATOLOGICAL ANALYSIS
Immediately upon collection, mixing of blood collection tubes by multiple manual inversions effected by jerking wrist movements insures that complete mixing of anticoagulant with blood has occurred.
Prior to analysis, complete homogenization of sample is effected in most laboratories by using a variety of mechanical mixers.
In the days prior to the availability of mechanical mixers there was even a debate among the old school of hematologists as to how many manual inversions effected by back-and-forth wrist movements were required for achieving homogeneity of blood samples.
The number that was thrown around to achieve complete homogenization of sample was approximately 20-25 manual inversions!
With the mechanical mixers one must be aware of the type of mixer that is used: rocking- versus rotary-type mixer. The blood collection tube should be examined to see if there is sufficient headspace available for mixing, especially when rocking-type mixers are used [1].
Rocking-type mixers are efficient when just enough headspace is present to effect mixing. However, if the blood collection tube is completely filled, mixing to homogeneity is prevented.
Thus in one study the hemoglobin value had doubled compared to the value obtained a week before, and the white blood cell (WBC) and platelet counts were significantly reduced.
However, after the analysis was repeated on the same specimen four times, results on hemoglobin, WBC and platelets were comparable to results obtained on the patient’s specimen a week before.
Apparently, the removal of aliquots of blood during four repeated analyses created sufficient headspace for the air bubble to move and achieve mixing to homogeneity on the rocking-type mixer [2].
Variability in mixing techniques is highlighted by the results obtained in a study comparing hemoglobin measurements performed by nurses using a manual procedure of mixing by rotating tubes by hand at the nurses station and by laboratory personnel in the laboratory using a rotary-type mixer [3].
Even though the same point-of-care (POC) analyzer was used at both the nurses station and the laboratory and the results were compared to those obtained with the routine laboratory’s mechanized analyzer, the correlation of results obtained by nurses, thoroughly trained to operate the POC analyzer, with the mechanized laboratory instrument was poor (r= 0.61) due to non-uniform and improper mixing.
In contrast, results for hemoglobin obtained by laboratory personnel on the POC analyzer correlated well with the mechanized laboratory instrument (r= 0.99) due to thorough homogenization of samples effected by mixing on a rotary-type mixer.
BLOOD GAS ANALYSIS
Pitfalls to avoid
1. Air bubbles
Immediately upon arterial blood collection in a syringe the
specimen should be checked to see if air bubbles are present.
If they are present, they should be expelled immediately either by applying the tip of the syringe to a piece of gauze or using a ventilated tip cap, if available, and tapping it while holding the syringe vertically.
This manipulation insures the removal of air bubbles. Once the air bubbles have been expelled, the needle is removed and safely discarded. The tip of the syringe is then sealed and mixed to effect complete dissolution of the anticoagulant (preferably dry balanced salt heparin) present in the syringe.
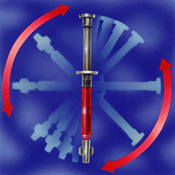
Mixing at the collection site should be done by inverting the syringe at least five times and rolling it between the palms for 5 seconds prior to analysis immediately [4].
Stored syringe specimens should be remixed by inverting the syringe 10 times and then rolling it horizontally between the palms for 10 seconds [4, 5] (Figures I and II).
Upon storage, specimens tend to settle and may require a longer mixing time to insure homogeneity of sample.
Specimens stored for longer periods, such as 30 minutes, may require mixing on a mechanical rotator for several minutes followed by repeatedly inverting the syringe and rolling it horizontally between the palms to achieve homogeneity.
In samples that have settled and not been thoroughly mixed, the hemoglobin value as discussed under hematological analysis can either be over- or underestimated depending on whether the concentrated cellular layer or plasma-diluted layer was measured.
 |
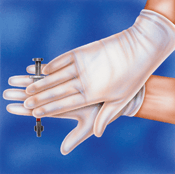 |
FIGURES I and II
The pO2 determination will be impacted most by the presence of air bubbles. In one study, a significant effect was noted both at 4 °C and at 22 °C. The effect was greater when multiple small bubbles were present as it increased the air/water surface, than when a single air bubble was present [6].
The longer the air bubble remains in the syringe and more the sample is disturbed, the greater is the effect on pO2 with the effect being noticeable just after 30 seconds.
Hence air bubbles should be expelled within 30 seconds of collection. One study noted a statistically significant change in pO2 value only 60 seconds after sample collection [7].
In this study, it was also noted that if the sample is left without removal of the air bubble for 30 minutes, the magnitude of change in the pO2 value was much greater.
In addition, a significant decrease in pCO2 was also seen with the air bubble remaining in the sample 30 minutes after collection.
The direction of change in the pO2 value (positive or negative) will be dependent on the initial pO2 value of the sample. Usually the bias will be positive since the normal pO2 value in arterial blood is 90-100 mmHg (12-13 kPa) in contrast to pO2 in atmospheric air, which is 150 mmHg (20 kPa).
However, samples with high pO2 values can yield artifactually lower measured values due to equilibration with the oxygen in the air bubble. The effect of air bubbles on samples stored on ice is greater due to the increased affinity of hemoglobin to oxygen at low temperature.
It has been reported that air bubbles with a relative volume of 0.5-1.0 % of the sample may cause significant errors [8].
In a clinical situation, therapy could be affected if the air bubble remains 30 minutes after sample collection with pO2 values increasing from a mean of 13.5 mmHg (1.8 kPa) to as much as 41.3 mmHg (5.5 kPa) [7].
2. Blood clots
Incomplete dissolution of anticoagulant in the syringe due to inadequate mixing immediately after collection can result in the formation of blood clots. The presence of microclots in the specimen renders it non-homogeneous, thus affecting the accuracy of analytical results.
Clots can also block the pathway of the blood gas analyzer and give either confounding results or even render the analyzer inoperable. Potassium results in blood gas analyzers that have the capability to also measure electrolytes can also be spuriously increased due to the lysis of some of the erythrocytes during the formation of the clot.
Hence it should be emphasized that thorough mixing of the blood specimen immediately upon collection is imperative to avoid formation of blood clots.
3. Hemolysis
Vigorous mixing of a blood sample can cause hemolysis. Blood collection artifacts, such as increased pressure through a narrow-diameter syringe or when the path of a syringe is blocked by microclots, can lead to hemolysis.
Either milking or squeezing the skin during capillary blood sampling or vigorous mixing using a metallic flea can cause hemolysis. Hemolysis can also occur during storage directly on ice or during transport in old pneumatic tube systems.
Hemolysis of just 0.1 % of erythrocytes may escape attention, making the sample virtually visually identical to a non-hemolyzed sample, but yet may contribute to an increased potassium value [9].
Hemolysis of even 1 % of erythrocytes can lead to a significant increase in potassium and a decrease in ionic calcium value [4]. The latter is due to a dilutional effect since the concentration of ionic calcium in cells is less when compared to that present in plasma.
The presence of hemolysis can be verified by centrifuging an aliquot of blood in a hematocrit tube and noticing whether the plasma layer is clear or has a reddish tinge. The latter condition will confirm the presence of hemolysis.
EFFECT OF THE TYPE OF SYRINGE AND STORAGE
CONDITIONS
Even a thoroughly mixed and homogeneous specimen can yield incorrect results due to variables introduced by not only the type of syringe but also storage conditions prior to performing analysis. In that context it is worthwhile to review briefly these variables.Due to the permeability of plastic materials to gases, such syringes (even high-density polypropylene syringes) are not perfectly gastight.
The design of the plastic syringe itself in terms of the fitting of plunger and stopper, surface-to-volume relationship and thickness of the wall material may dictate the extent of shift in pO2 and pCO2 values from the true value [4].
Thus in one study it was shown that not all plastic syringes will bias the pO2 value to the same extent [10]. In this study, it was shown that the increase in pO2 was greater in a 1-mL syringe when compared to a 2-mL syringe.
Since changes in pO2 were demonstrated 20 minutes after collection, with significant changes noted after 45 minutes of storage in an ice-water bath, it was recommended that blood collected in plastic syringes should be analyzed within 15 minutes of collection [10].
If this cannot be done, the use of glass syringes was recommended.
Glass syringes stored on ice-water slush for even up to 2 hours may yield acceptable results for blood gas analysis [4].
However, measurement of potassium will be affected beyond 1 hour of storage since refrigeration affects the sodium-potassium ATPase pump, causing potassium to leak out of the cells [9].
NCCLS recommends that plastic syringes containing blood for the purpose of blood gas and/or electrolyte analysis should not be iced but kept at room temperature and should be analyzed within 30 minutes of collection [11].
In contrast to storage at room temperature, the rate of increase in pO2 value in specimens collected in plastic syringes and stored on ice has been attributed to the influx of exogenous oxygen through the walls of the plastic syringe.
Actually, the metabolic consumption of oxygen is decreased at low temperature. This influx of oxygen into the plastic occurs due to the very high concentration of oxygen in ice water [12, 13].
In contrast, glass syringes are impermeable to gases and any decrease in pO2 value on storage is attributable to cellular metabolism, which is slowed at the low temperature of storage on ice.
The extent of increase in pO2 in samples stored in plastic syringes on ice is dependent on the oxygen-buffering capacity of hemoglobin. Thus samples with an initial pO2 value of less than 50 mmHg showed a lesser increase over time in samples stored on ice when compared to samples with pO2 values in the 50-250 mmHg range [12].
For samples with pO2 values in excess of 200 mmHg (27 kPa), the use of glass syringes is recommended [4]. Indeed, the limitation of even high-density plastic (polypropylene) syringes for analysis of samples with high pO2 values (at levels where hemoglobin is fully saturated) was shown in one study [14].
In this study, a rapid fall in oxygen tension was shown in the first 10 minutes of storage at room temperature.
Thus the average rate of decrease in the first 10 minutes was 9.1 mmHg/min (1.21 kPa/min) in plastic syringes compared to 3.7 mmHg/min (0.49 kPa/min) in samples collected in glass syringes. Storage on ice, however, decreases the rate of loss.
Glass syringes are preferred for the analysis of samples with high pO2 values, and even such syringes should be stored on ice and analyzed as soon as possible [11].
Storage of plastic syringes even for short periods at room temperature can result in significant errors in estimating shunt fraction by the 100 % oxygen technique, with each minute delay from blood sampling to analysis, on an average, overestimating the shunt value by 0.6 % [14].
MIXING CAPILLARY BLOOD
The procedure for mixing arterial blood collected in blood gas syringes has already been discussed. In addition to the manual mixing procedure, which entails inversion of the syringe and rolling it between the palms, arterial blood gas samplers containing a metal ball to facilitate mixing may also be used.The procedure for mixing capillary blood involves using a metal mixing bar or flea and a magnet. Even when the mixing procedure is thorough, errors will occur if attention is not paid to the preanalytical aspects of capillary blood collection.
Hence it is appropriate to briefly recapitulate the manipulations involved in capillary blood sampling. Capillary blood is collected by skin puncture with a lancet after the site has been warmed with a hot moist towel for 3 minutes at a temperature ranging from 39 to 42 °C to arterialize the area [4].
After the first drop of blood has been discarded, blood is allowed to flow into a heparinized capillary without any squeezing of the skin.
The tip of the capillary is placed deeply into the drop while holding the capillary in a horizontal or slightly downward position, taking care not to introduce air bubbles. After the capillary tube has filled completely, the end of the capillary that was in the blood drop is closed off immediately with a plastic cap.
Subsequently, a metal mixing bar or flea is inserted. The opposite end of the capillary is then sealed. Blood and heparin in the capillary tube are mixed by moving the metal bar with the help of a magnet five times from end to end.
This procedure will insure thorough mixing and prevent formation of blood clots [4, 5].
SUMMARY
Samples that are thoroughly mixed and rendered homogeneous are a prerequisite for hematological and blood gas analysis. Preanalytical variables prior to mixing need to be controlled.
The key to the validity of results in blood gas analysis of even a well-mixed specimen free of preanalytical artifacts is to preferably analyze the sample promptly upon collection.
This will minimize changes that may occur upon storage in specimens from very sick patients who may have conditions such as thrombocytosis, leukocytosis or anemia.
References+ View more
- Narayanan S. Preanalytical issues in hematology. J Lab Med 2003; 27: 243-48.
- Pewarchuk W, Vanderbroom J, Blacjchman MA. Pseudopolycythemia, pseudothrombocytopenia, and pseudoleukopenia due to overfilling of blood collection vacuum tubes. Arch Pathol Lab Med 1992; 116: 90-92.
- Neville RG. Evaluation of portable hemoglobinometer in general practice. Br J Med 1987; 294: 1263-67.
- Burnett RW, Covington AK, Fogh-Andersen N, et al. International Federation of Clinical Chemistry (IFCC), Approved IFCC recommendations on whole blood sampling, Transport and Storage for simultaneous determination of pH, Blood gases and electrolytes. Eur J Clin Chem Clin Biochem 1995; 33: 247-53.
- Guder WG, Narayanan S, Wisser H, et al. Samples: From the patient to the laboratory. 3rd revised ed. Darmstadt: Wiley-VCH, 2003.
- Clark CG, Rayfield JM, Clague AE. Preanalytical errors in simultaneous blood gas, electrolyte and metabolite analysis. Blood Gas News 1998; 7 (1); 12-15.
- Harsten A, Berg B, Inerot S, Muth L. Importance of correct handling of samples for the results of blood gas analysis. Acta Anaesthesiol Scand 1988; 32: 365-68.
- Mueller RG, Lang GE, Beam JM. Bubbles in samples for blood gas determinations – a potential source of error. Am J Clin Pathol 1976; 65: 242-49.
- Narayanan S. The preanalytic phase. An important component of laboratory medicine. Am J Clin Pathol 2000; 113; 429-52.
- Muller-Plathe O, Heyduck S. Stability of blood gas, electrolytes and hemoglobin in heparinized whole blood samples: Influence of the type of syringe. Eur J Clin Chem Clin Biochem 1992; 30: 349-55.
- Blonshine S, Alberti R, Olesinki, RL. Procedures for the collection of arterial blood specimens; Approved standard – third edition, NCCLS document H11-A3. Wayne, Pa: NCCLS, 1999: 12, 8.
- Beaulieu M, Lapointe Y, Vinet B. Stability of pO2, pCO2 and pH in fresh blood samples stored in a plastic syringe with low heparin in relation to various blood-gas and hematological parameters. Clin Biochem 1999; 32: 101-07.
- Wu EY, Barazanji KW, Johnson RL. Source of error on A-a pO2 calculated from blood stored in plastic and glass syringes. J Appl Physiol 1997; 82: 196-202.
- Pretto JJ, Rochford PD. Effects of sample storage time, temperature and syringe type on blood gas tensions in samples with high oxygen partial pressures. Thorax 1994; 49: 610-12.
References
- Narayanan S. Preanalytical issues in hematology. J Lab Med 2003; 27: 243-48.
- Pewarchuk W, Vanderbroom J, Blacjchman MA. Pseudopolycythemia, pseudothrombocytopenia, and pseudoleukopenia due to overfilling of blood collection vacuum tubes. Arch Pathol Lab Med 1992; 116: 90-92.
- Neville RG. Evaluation of portable hemoglobinometer in general practice. Br J Med 1987; 294: 1263-67.
- Burnett RW, Covington AK, Fogh-Andersen N, et al. International Federation of Clinical Chemistry (IFCC), Approved IFCC recommendations on whole blood sampling, Transport and Storage for simultaneous determination of pH, Blood gases and electrolytes. Eur J Clin Chem Clin Biochem 1995; 33: 247-53.
- Guder WG, Narayanan S, Wisser H, et al. Samples: From the patient to the laboratory. 3rd revised ed. Darmstadt: Wiley-VCH, 2003.
- Clark CG, Rayfield JM, Clague AE. Preanalytical errors in simultaneous blood gas, electrolyte and metabolite analysis. Blood Gas News 1998; 7 (1); 12-15.
- Harsten A, Berg B, Inerot S, Muth L. Importance of correct handling of samples for the results of blood gas analysis. Acta Anaesthesiol Scand 1988; 32: 365-68.
- Mueller RG, Lang GE, Beam JM. Bubbles in samples for blood gas determinations – a potential source of error. Am J Clin Pathol 1976; 65: 242-49.
- Narayanan S. The preanalytic phase. An important component of laboratory medicine. Am J Clin Pathol 2000; 113; 429-52.
- Muller-Plathe O, Heyduck S. Stability of blood gas, electrolytes and hemoglobin in heparinized whole blood samples: Influence of the type of syringe. Eur J Clin Chem Clin Biochem 1992; 30: 349-55.
- Blonshine S, Alberti R, Olesinki, RL. Procedures for the collection of arterial blood specimens; Approved standard – third edition, NCCLS document H11-A3. Wayne, Pa: NCCLS, 1999: 12, 8.
- Beaulieu M, Lapointe Y, Vinet B. Stability of pO2, pCO2 and pH in fresh blood samples stored in a plastic syringe with low heparin in relation to various blood-gas and hematological parameters. Clin Biochem 1999; 32: 101-07.
- Wu EY, Barazanji KW, Johnson RL. Source of error on A-a pO2 calculated from blood stored in plastic and glass syringes. J Appl Physiol 1997; 82: 196-202.
- Pretto JJ, Rochford PD. Effects of sample storage time, temperature and syringe type on blood gas tensions in samples with high oxygen partial pressures. Thorax 1994; 49: 610-12.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Minimizing Pre-analytical errors in blood gas testing
Presented by Ana-Maria Simundic, PhD, Prof. of Medical Biochemistry, Zagreb University, Zagreb, Croatia Watch the webinarScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars