Printed from acutecaretesting.org
September 2003
Principles and problems of blood glucose measurement
Introduction
The measurement of glucose is one of the longest established and most frequently performed tests in the clinical biochemistry laboratory. Surprisingly, despite the availability of purified reference standards, calibration of blood glucose methods can be extremely complex and, in some cases, rather approximate.
This often stems from the fact that different techniques assay the glucose present in different fractions of the blood sample. They may employ different analytical principles to do this and may even express the results in a different way.
I shall use the term blood glucose in this article in a general sense to mean glucose in any blood fraction or fractions, such as plasma or whole blood.
When I wish to be more specific, I shall state the fraction(s) involved.
Situations in which blood glucose assays are performed
In the hospital laboratory, it is usual to perform glucose assays on plasma or serum, as routine venepuncture and the availability of centrifuges usually mean that sufficient plasma can be harvested for assay on the main laboratory analyzer.
In other situations, such as intensive care units or special care baby units, it is less convenient to use plasma, either because of lack of centrifuge facilities or owing to small sample volume.
Under such circumstances, it is preferable to be able to present whole blood to the analytical system, as it is in a third type of scenario, where the patient performs the assay himself.
Assay methods
Nowadays, chemical methods for blood glucose assay invariably rely upon stages involving enzymes (e.g. glucose oxidase, glucose dehydrogenase, hexokinase, etc.) linked to chromogenic reactions or to reactions featuring changes in electron flow that can be measured by suitable electronic meters.
There are also techniques, less widely available, that employ physical methods for glucose detection, such as differences in infrared spectra.
At first it seems strange that laboratory techniques should be the simplest, most straightforward types of blood glucose assay in use, whereas some of the near-patient or point-of-care methods are really quite complicated in principle, but this is largely due to the constraints placed upon the latter by the requirement to be able to measure the glucose in a non-homogeneous matrix, i.e. whole blood.
Do all blood glucose assays measure the same thing?
The simple answer is, "No".
Having said that, laboratory methods using plasma, essentially a homogeneous matrix, do generally agree quite well because the assay responds to the glucose dissolved in the entire volume of the sample and results are usually expressed in terms of concentration of glucose per unit volume of plasma, e.g. in mmol/L.
For methods using whole blood, the situation is very different and partly depends on whether the blood sample is first hemolyzed or diluted in some way before the measurement is performed.
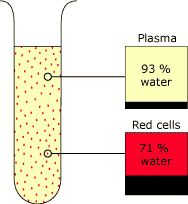
Understanding this depends on the knowledge that red cells and plasma contain different amounts of dissolved solids such as proteins and, hence, have different proportions of water per unit volume, the water content of a volume of red cells being lower than that of an equal volume of plasma (Table I and Fig. 1).
Glucose is dissolved in the water of the specimen (ignoring any bound to proteins) and equilibrates freely between the red cells and plasma of a whole-blood specimen, so the concentrations in plasma water and red-cell water are the same, but the concentration in total red-cell contents is lower than in plasma and the concentration in a volume of whole blood lies somewhere in between, varying with the hematocrit of the specimen (Table II).
Consequently, the glucose concentration influencing the assay system will be determined by whether the assay responds to the concentration in the water, in the plasma, in the red cells, in a mixture of plasma and red cells, or in a dilution of the blood specimen.
|
Plasma |
Red cells |
Whole blood with different hematocrit (Hct) levels |
|||
|
Hct 0.3
|
Hct 0.45 | Hct 0.6 | |||
|
Water content (%) |
93 |
71 |
86 |
83 |
80 |
TABLE I and FIG. 1. Approximate water content
of blood fractions expressed as a percentage of total volume.
| |
Plasma |
Red cells |
Whole blood with different hematocrit (Hct) levels |
||
|
Hct 0.3
|
Hct 0.45 | Hct 0.6 | |||
|
Water content (%) |
93 |
71 |
86 |
83 |
80 |
|
Glucose
|
4.8 |
3.6 |
4.3 |
4.2 |
4.0 |
TABLE II. Differing glucose concentrations of
plasma, red cells and whole-blood specimens when glucose molality
of all samples is the same at 5 mmol/kg H2O.
The sophisticated principles involved in many point-of-care glucose methods make it difficult to know exactly which fraction of the sample is influencing the method's response, but in those cases where a filtering process takes place, retaining the red cells and allowing the plasma to seep through into the reagent area, it appears safe to assume that it is only the plasma glucose that is being measured.
However, the situation may not be quite so simple in that blood samples with different proportions of red cells may influence the flow and volume of plasma entering the reagent layers, and thus may affect the result.
In methods where the whole-blood sample is in contact with the reagents, there can still be interference from hematocrit, and even those techniques where the red cells are lyzed, releasing red-cell contents into the reaction milieu, are potentially subject to influence by the different glucose concentrations of plasma and red cells.
Some laboratory instruments capable of accepting a whole-blood sample (e.g. YSI) involve diluting it without lysis of red cells before the measurement takes place, using a glucose oxidase-linked electrode system.
This has the effect of withdrawing some of the glucose from the red cells in an attempt to establish a new equilibrium and consequently, the final result is in terms of glucose concentration in the whole-blood specimen.
The result is affected by the hematocrit because the more red cells there are present with lower water, and hence glucose content, than in an equal volume of plasma, the more the result will be lowered by the effect of the dilution.
On the other hand, in direct-reading electrode methods, such as are usually employed on blood gas analysis systems, where no dilution of the blood takes place, the electrode responds to the activity of the glucose in the water of the specimen, which is equivalent to the molality in mmol/kg water.
In theory, this should not be affected by hematocrit [1].
As a simple rule, one could say that systems in which there is dilution of the blood sample are influenced by the total glucose and water content of the sample, whereas systems measuring glucose directly on undiluted whole blood are only affected by the activity or molality of glucose in the water of the sample.
Calibration of methods
While the method principle is an important determinant of the final result of the assay, the method of calibration is of equal, or perhaps even greater, importance.
Some methods, e.g. the YSI, employ aqueous glucose standards, but manufacturers of point-of-care assays usually set up their systems so that a blood sample will give approximately the same result on their analyzer as it will by their chosen "reference" method.
This assumes, however, that all specimens have similar characteristics and that the method under calibration will react in the same way as the reference method in all circumstances. In practice, we know that this is not always the case.
For example, while an assay may give the same result as the reference method for a sample of average hematocrit, results may diverge when the hematocrit is high or low owing to factors that are separate from, and additional to, the effects of different water distribution in the different blood fractions.
How should results be expressed?
There is a legitimate argument that glucose results should be expressed in terms of activity in the water of the specimen (numerically regarded as the same for plasma and red cells), because this represents the glucose available at the tissues.However, most current laboratory methods do not directly measure this. Instead, they measure concentration in plasma, which is numerically lower, and this is how results are usually expressed.
For methods using a whole-blood sample the result is often, but not invariably, expressed as whole-blood glucose concentration, giving a figure lower than that for plasma glucose.
Conventions vary in different countries, the argument for expression as whole-blood glucose concentration being that the sample is actually whole blood. Against this is the argument that expression as plasma glucose allows comparison with laboratory results.
In truth neither is entirely correct, as some assays will be influenced by the presence of varying amounts of red cells in different samples.
Assumptions have, therefore, to be made by the manufacturers about the average red-cell content of the blood sample in the knowledge that deviations from this will introduce a degree of error.
What is important to realize, however, is that a method accepting a whole-blood sample cannot be presumed to be expressing results in a particular way without careful checking of the manufacturer's literature.
Even the direct-reading electrode systems usually factorize the results to express them in conventional concentration terms, rather than as the activity actually measured, and it has been proposed that all methods should report as plasma glucose concentration to avoid confusion, no matter what sample type or measurement method has been used [2,3].
While this may be satisfactory for direct-reading electrode systems, it was not found to be entirely reliable for ear-prick capillary blood hemolysates assayed by a glucose dehydrogenase method [4].
Preanalytical considerations
Of course, the results of a glucose estimation are not only determined by the analysis itself, but also by preanalytical factors. It is well-known that fluoride, commonly used as an inhibitor of glycolysis in blood specimens, is not entirely effective and that a slow degradation of glucose occurs, especially in the first couple of hours after collection [5].
Even the oxygen content of the blood sample can influence some methods, such that they have to be differently calibrated specifically for venous or capillary blood [6].
The site and timing of blood sampling are also important. For example, although venous and capillary blood samples collected from a fasting subject usually give similar results, postprandially a capillary sample would give a higher glucose result, owing to uptake of recently absorbed glucose by the tissues.
Realization of this sort of phenomenon is becoming more important now that manufacturers of point-of-care systems are promoting, or at least facilitating, alternative-site testing.
There is a perception that some patients wish to avoid finger-pricking and would prefer to collect capillary blood from other areas of their bodies, such as other parts of the hand, forearm or leg.
The difficulty with this is that changes in blood glucose in these areas appear to lag behind those in venous or finger-prick capillary blood. Consequently, a patient may become symptomatically hypoglycemic before a blood sample from one of these alternative sites shows a low glucose level.
Conversely, hyperglycemia may not be detectable as soon as conventional sampling would allow.
For this reason it is recommended that alternative-site sampling is used only when the subject is glycemically in steady state, i.e. when fasting or at least two hours after food and not postprandially or after administration of insulin.
Conclusion
The measurement of blood glucose is made surprisingly complex by affording the user the convenience of being able to present a whole-blood sample to the analytical system. This leads to uncertainty about which part of the glucose of the specimen is being measured.
It involves making assumptions, compromising scientific principles and often expressing the result in a form different from the way it has been assayed.
However, given the very different analytical techniques being employed in these methods, there is at present no easy way of bringing them all into alignment to achieve true comparability, although an agreement to standardize the calibration of direct-reading electrode systems would go some way towards this.
Nevertheless, the problem is likely to remain with us for some time to come and preanalytical variables such as site of blood sampling can also add to the complications.
References+ View more
- Fogh-Andersen N, Wimberley PD, Thode J, Siggaard-Andersen O. Direct reading glucose electrodes detect the molality of glucose in plasma and whole blood. Clin Chim Acta 1990; 189: 33-38.
- Fogh-Andersen N, D'Orazio P. Proposal for standardizing direct-reading biosensors for blood glucose. Clin Chem 1998; 44: 655-59.
- Fogh-Andersen N, D'Orazio P, Kuwa K, Külpmann WR, Mager G, Larsson L. Recommendation on reporting results for blood glucose (From an IFCC Stage 1 document) IFCC Scientific Division Working Group on Selective Electrodes. EJIFCC, Vol 12 No 4; http://www.ifcc.org/ejifcc/vol2no4/vol12no4a4.htm
- Stahl M, Brandslund I, Jørgensen LGM, Hyltoft Petersen P, Borch-Johnsen K, De Fine Olivarius N. Can capillary whole blood glucose and venous plasma glucose measurements be used interchangeably in diagnosis of diabetes mellitus? Scand J Clin Lab Invest 2002; 62: 159-66.
- De Pasqua A, Mattock MB, Phillips R, Keen H. Errors in blood glucose determination. Lancet 1984; ii: 1165.
- Halloran SP. Influence of blood oxygen tension on dipstick glucose determinations. Clin Chem 1989; 35: 1268-69.
References
- Fogh-Andersen N, Wimberley PD, Thode J, Siggaard-Andersen O. Direct reading glucose electrodes detect the molality of glucose in plasma and whole blood. Clin Chim Acta 1990; 189: 33-38.
- Fogh-Andersen N, D'Orazio P. Proposal for standardizing direct-reading biosensors for blood glucose. Clin Chem 1998; 44: 655-59.
- Fogh-Andersen N, D'Orazio P, Kuwa K, Külpmann WR, Mager G, Larsson L. Recommendation on reporting results for blood glucose (From an IFCC Stage 1 document) IFCC Scientific Division Working Group on Selective Electrodes. EJIFCC, Vol 12 No 4; http://www.ifcc.org/ejifcc/vol2no4/vol12no4a4.htm
- Stahl M, Brandslund I, Jørgensen LGM, Hyltoft Petersen P, Borch-Johnsen K, De Fine Olivarius N. Can capillary whole blood glucose and venous plasma glucose measurements be used interchangeably in diagnosis of diabetes mellitus? Scand J Clin Lab Invest 2002; 62: 159-66.
- De Pasqua A, Mattock MB, Phillips R, Keen H. Errors in blood glucose determination. Lancet 1984; ii: 1165.
- Halloran SP. Influence of blood oxygen tension on dipstick glucose determinations. Clin Chem 1989; 35: 1268-69.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








