Printed from acutecaretesting.org
October 2005
Reducing phlebotomy blood loss in the NICU
ANEMIC PATIENTS IN THE NICU ARE AMONG THE MOST HIGHLY TRANSFUSED PATIENT GROUPS
Critically ill newborn infants are among the most heavily transfused groups [1]. Despite advances in the understanding of the pathophysiology and treatment of neonatal anemia, RBC transfusion continues to be the primary treatment for this commonly encountered condition.
Reducing blood transfusions is desirable, since they increase the risk of iatrogenic infection and adverse reactions. Although the use of increasingly restrictive transfusion criteria for neonates has been successful in reducing RBC transfusions [1, 2], still an estimated 1,000,000 transfusions are administered annually to neonates in the US [2, 3].
Approximately 60-80 % of very-low-birth-weight (VLBW) infants (i.e., with birth weights <1,500 g) currently receive one or more RBC transfusions prior to hospital discharge as treatment for clinically significant anemia [4].
Although less well recognized, RBC transfusion of larger infants is also common, accounting for up to 45 % of all transfusions in the NICU [5].
LABORATORY BLOOD LOSS IN NICU: THE PRIMARY CAUSE OF ANEMIA AND THE NEED FOR RBC TRANSFUSIONS
There is strong agreement that the primary cause of neonatal anemia is iatrogenic phlebotomy loss resulting from the intensive monitoring which critically ill infants receive in the weeks immediately following birth [2, 3, 6].Sampling loss in the NICU is directly associated with low gestational age and severity of illness [7]. Studies have shown that daily phlebotomy blood loss of 4-5 % of an infant’s 80 mL/kg blood volume during the early neonatal period is not uncommon among the sickest infants [8, 9].
When examined on a weekly basis for only premature infants, laboratory blood loss averages 15-30 % of total blood volume every week for the first four weeks of life [3].
The volume of blood transfused in the NICU has been directly related to the volume of blood removed – in some cases on a 1-to-1, milliliter-for-milliliter basis [8-10].
In many [4] but not all [11] NICUs, approximately 50 % of all RBC transfusions administered to VLBW infants are given in the first two weeks of life, with 70 % administered by the first month [4], although iatrogenic blood loss continues to contribute to transfusion requirements beyond this early neonatal period.
ADVANCES IN LABORATORY BLOOD TESTING HAVE REDUCED ANEMIA AND TRANSFUSION IN THE NICU
Clearly, to reduce RBC transfusion in the NICU, new approaches to reducing iatrogenic blood loss for laboratory testing must be considered. It has been suggested that iatrogenic blood loss in neonates can be reduced by restricting blood testing to only that which is most essential [1, 2, 6].This seemingly common-sense approach to preventing neonatal anemia is complicated by the fact that there is no consensus as to what constitutes “essential” testing. Moreover, there are no experimental data to show that this practice is either effective or safe.
In contrast, the technical improvements in the benchtop “analyzers” (instruments requiring ever-diminishing blood volumes) and the highly accurate bedside, point‑of‑care “monitors” (instruments that return the analyzed blood to the patient) described below have led to significant decreases in neonatal blood loss [12, 13].
Indeed, improvements in instrumentation in combination with the application of more restrictive RBC transfusion criteria are the primary reasons for the reduction in RBC transfusions reported for VLBW infants [1, 2].
SPECIFIC BLOOD TESTS COMMONLY ORDERED IN THE NICU FOR CRITICALLY ILL INFANTS
What do we know about the kinds and frequency of blood testing in the NICU? This information would be helpful in designing effective strategies for reducing iatrogenic neonatal blood loss.For all critically ill patients – including neonates – repeated measurement of blood gases, electrolytes, glucose and hemoglobin concentration from indwelling arterial or central venous vascular catheters remains a cornerstone of the intensive care that such individuals require.
To determine the number and kinds of laboratory blood tests typically performed on critically ill VLBW infants with indwelling umbilical arterial catheters in the first weeks of life, we retrospectively examined the specific repetitive laboratory tests that a group of 50 VLBW infants experienced [14].
As illustrated in Figure I, the number of laboratory blood tests performed during the first week of life while arterial catheters were available for blood sampling averaged ~20 per infant per day.
The most commonly performed blood tests in our NICU were blood gases and electrolytes, followed in decreasing order by glucose, hemoglobin/hematocrit, total bilirubin and calcium.
Hence, reducing iatrogenic neonatal loss should primarily focus on reducing the blood required for performing these tests.
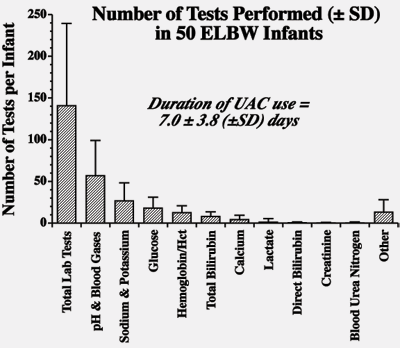
FIGURE I: Average number of blood tests performed per VLBW infant while critically ill and during the period when arterial catheters were in use (n = 50). (Modified from Alves-Dunkerson et al. [14].
TECHNICAL SOLUTIONS FOR REDUCING BLOOD LOSS IN THE NEONATE
1. Central-laboratory instruments
Core or central laboratories are not equipped to minimize blood loss in the neonate. These laboratories are focused on high-volume testing drawn from adults in standardized vacuum tubes.
Samples that cannot be processed by automated equipment, such as pediatric microtubes, must be handled manually. Most chemistry tests, which utilize serum or plasma, require relatively large volumes of whole blood due to the poor yield of high-hematocrit specimens from neonates. Automated analyzers have substantial “dead volume” requirements for adequate sampling.
This means that even if testing can be performed on small sample volumes, additional blood must be drawn to insure adequate sampling [15].
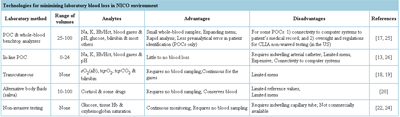
One solution that has successfully been applied to the problem of how to reduce the volume of blood needed for testing in neonates is the use of blood bas analysers, e.g., benchtop instruments capable of analyzing whole blood instead of plasma or serum (see Table I).
Expanding the capabilities of these instruments by including additional tests such as electrolytes, lactate, bilirubin, etc. has resulted in little-to-no increase in sample volume [16].
TABLE 1: Click on the image to see the table
2. Point-of-care-testing (POCT) analyzers and monitors
Like benchtop blood gas analyzers in the central core laboratory, modern POC devices including “analyzers” (which permanently remove blood) and in-line “monitors” (which return the analyzed blood to the patient) are also capable of analyzing whole-blood samples in small quantities.
Since with POC devices all steps in the processing and handling (including specimen labeling) of samples can be performed at the bedside by a single individual, an important feature of the POC devices is that preanalytical error is less than for core laboratory analyzers [17].
In addition to conserving specimen, POC testing permits rapid decision making as a result of immediate access to test results.
The menus available for POC analyzers – but not yet for monitors – are substantial and growing, and include many of the most commonly ordered tests included in the Figure.
POC testing is not a perfect solution. Costs are usually higher compared with testing performed in a central lab, and the burden of POC testing often falls on the nursing staff, who are already coping with multiple, complex patient care duties and who require formal training in the use of the equipment.
However, quality test results are achievable through oversight and partnership with the laboratory, and the increased cost may be more than offset by the prospect of better patient outcomes.
3. Transcutaneous measuring devices
Transcutaneous measurements will decrease the need for blood samples.
The thin, translucent skin of newborns makes the measurement of some analytes easier than in adults [18]. For example, measurement of pO2 and pCO2 are available transcutaneously and have been shown to correlate with assays performed on a blood gas analyzer [19].
Despite this advantage, the use of transcutaneous measurements has declined following the introduction of pulse oximetry SpO2 measurement. Although pulse oximetry is inaccurate at high pO2 levels, it is more user-friendly for the nurse and technician and it does not carry the risks of thermal injury.
More recently, reliable transcutaneous measurement of bilirubin has become possible. Several meters are commercially available that have demonstrated good agreement of serum and transcutaneous bilirubin measurements.
As with all laboratory testing, proper attention to details such as calibration and ongoing comparisons with the serum method is essential.
Thus far, transcutaneous bilirubin measurement has been reserved primarily for screening purposes; transcutaneous bilirubin levels that require clinical intervention should still be confirmed on a blood sample using a chemical method.
4. Use of alternative specimens
Saliva and interstitial fluid are the two most studied – yet underutilized – alternative specimen types. Saliva has proven feasible and reliable for the measurement of hormones such as cortisol and some commonly administered neonatal drugs, e.g., theophylline and caffeine [20].
Specialized devices to collect both types of fluid are available [21].
Problems include sensitivity of assays used, since the concentration of some analytes in saliva may be far less than in blood. For this reason, saliva specimens must be free of blood. Reference ranges for saliva are less well established than those for blood, especially for neonates.
5. Future technologies
Non-invasive measurement of analytes in the circulation has long been a goal of researchers and clinicians. Infrared spectrophotometric methods for non-invasive glucose, tissue Hb and oxyhemoglobin saturation determinations have been described for some time [22, 23] and are likely to come on the market in the future.
In a few years, microchip, microarray and nanotechnology devices performing PCR, immunoassay and other analytical procedures on sub-microliter samples will also become available [24].
This technology will open up whole new areas for diagnosis and treatment of the neonate. Finally, a new POC blood counter capable of performing hemoglobin, hematocrit, WBC and three-point differential on 20 µL of capillary blood (as opposed to large hematology analyzers requiring 10 times this amount) is available in Europe and has been submitted to the FDA for approval.
In conclusion, advances in laboratory technology will continue the trend of allowing for the determination of more analytes on smaller and smaller volumes of blood.
Reducing the transfusion needs of critically ill neonates will improve their treatment by preventing the development of clinically significant anemia attributable to laboratory phlebotomy loss.
References+ View more
- Luban NLC. Neonatal red blood cell transfusions. Vox Sang 2004;87 Suppl 2:184-88.
- Ohls RK. Erythropoietin treatment in extremely low birth weight infants: blood in versus blood out. Journal of Pediatrics 2002;141:3-6.
- Widness JA. Pathophysiology, diagnosis and prevention of neonatal anemia. NeoReviews 2000;1:e61-e68.
- Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. J Pediatr 1996;129(5):680-87.
- Schmidt NM, Widness JA. Red blood cell transfusion practice and donor exposure among newborns weighing >1500 g at birth. Pediatric Research 2002;51:418A.
- Bifano EM. Traditional and nontraditional approaches to the prevention and treatment of neonatal anemia. NeoReviews 2000;1:e69-e73.
- Brune T, Garritsen H, Witteler R, Schlake A, Wullenweber J, Louwen F, et al. Autologous placental blood transfusion for the therapy of anaemic neonates. Biol Neonate 2002;81(4):236-43.
- Blanchette V, Zipursky A. Assessment of anemia in newborn infants. Clinics in Perinatology 1984;11:489-516.
- Obladen M, Sachsenweger M, Stahnke M. Blood sampling in very low birth weight infants receiving different levels of intensive care. European Journal of Pediatrics 1988;147:399-404.
- Shannon KM, Keith JF 3rd, Mentzer WC, Ehrenkranz RA, Brown MS, Widness JA, et al. Recombinant human erythropoietin stimulates erythropoiesis and reduces erythrocyte transfusions in very low birth weight preterm infants. Pediatrics 1995;95(1):1-8.
- Eichler H, Schaible T, Richter E, Zieger W, Voller K, Leveringhaus A, et al. Cord blood as a source of autologous RBCs for transfusion to preterm infants. Transfusion 2000;40(9):1111-17.
- Madan A, Kumar R, Adams MM, Benitz WE, Geaghan SM, Widness JA. Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. J Perinatol 2005;25(1):21-25.
- Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics 2005;115:1299-306.
- Alves-Dunkerson JA, Hilsenrath PE, Cress GA, Widness JA. Cost analysis of a neonatal point-of-care monitor. Am J Clin Pathol 2002;117:809-18.
- Lin JC, Strauss RG, Kulhavy JC, Johnson KJ, Zimmerman MB, Cress GA, et al. Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics 2000;106(2):E19.
- Peake M, Mazzachi B, Fudge A, Bais R. Bilirubin measured on a blood gas analyser: a suitable alternative for near-patient assessment of neonatal jaundice? Ann Clin Biochem 2001;38:533-40.
- Papadea C, Foster J, Grant S, Ballard SA, Cate JC, Southgate WM, et al. Evaluation of the i-STAT portable clinical analyzer for point-of-care blood testing in the intensive care units of a university children's hospital. Annals of Clinical & Laboratory Science 2002;32:231-43.
- DeNicola LK, Kissoon N, Abram HSJ, Sullivan KJ, Delgado-Corcoran C, Taylor C. Noninvasive monitoring in the pediatric intensive care unit. Pediatric Clinics of North America 2001;48:573-88.
- Carter BG, Wiwczaruk D, Hochmann M, Osborne A, Henning R. Performance of transcutaneous PCO2 and pulse oximetry monitors in newborns and infants after cardiac surgery. Anaesth Intensive Care 2001;29:260-65.
- Hofman LF. Human saliva as a diagnostic specimen. J Nutr 2001;131:1621S-1625S.
- Holm-Hansen C, Tong G, Davis C, Abrams WR, Malamud D. Comparison of oral fluid collectors for use in a rapid point-of-care diagnostic device. Clinical and Diagnostic Laboratory Immunology 2004;11:909-12.
- Khalil OS. Non-invasive glucose measurement technologies: an update from 1999 to the dawn of the new millennium. Diabetes Technology & Therapeutics 2004;6:660-97.
- Siegemund M, van Bommel J, Ince C. Assessment of regional tissue oxygenation. Intensive Care Med 1999;25(10):1044-60.
- Kricka LJ. Microchips, microarrays, biochips and nanochips: personal laboratories for the 21st century. Clinica Chimica Acta 2001;307:219-23.
- Meier FA, Jones BA. Point-of-care testing error: sources and amplifiers, taxonomy, prevention strategies, and detection monitors. Arch Pathol Lab Med 2005;129:1262-67.
- Kost GJ, Ehrmeyer SS, Chernow B, Winkelman JW, Zaloga GP, Dellinger RP, et al. The laboratory-clinical interface. Point-of-care testing. Chest 1999;115:1140-54.
References
- Luban NLC. Neonatal red blood cell transfusions. Vox Sang 2004;87 Suppl 2:184-88.
- Ohls RK. Erythropoietin treatment in extremely low birth weight infants: blood in versus blood out. Journal of Pediatrics 2002;141:3-6.
- Widness JA. Pathophysiology, diagnosis and prevention of neonatal anemia. NeoReviews 2000;1:e61-e68.
- Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. J Pediatr 1996;129(5):680-87.
- Schmidt NM, Widness JA. Red blood cell transfusion practice and donor exposure among newborns weighing >1500 g at birth. Pediatric Research 2002;51:418A.
- Bifano EM. Traditional and nontraditional approaches to the prevention and treatment of neonatal anemia. NeoReviews 2000;1:e69-e73.
- Brune T, Garritsen H, Witteler R, Schlake A, Wullenweber J, Louwen F, et al. Autologous placental blood transfusion for the therapy of anaemic neonates. Biol Neonate 2002;81(4):236-43.
- Blanchette V, Zipursky A. Assessment of anemia in newborn infants. Clinics in Perinatology 1984;11:489-516.
- Obladen M, Sachsenweger M, Stahnke M. Blood sampling in very low birth weight infants receiving different levels of intensive care. European Journal of Pediatrics 1988;147:399-404.
- Shannon KM, Keith JF 3rd, Mentzer WC, Ehrenkranz RA, Brown MS, Widness JA, et al. Recombinant human erythropoietin stimulates erythropoiesis and reduces erythrocyte transfusions in very low birth weight preterm infants. Pediatrics 1995;95(1):1-8.
- Eichler H, Schaible T, Richter E, Zieger W, Voller K, Leveringhaus A, et al. Cord blood as a source of autologous RBCs for transfusion to preterm infants. Transfusion 2000;40(9):1111-17.
- Madan A, Kumar R, Adams MM, Benitz WE, Geaghan SM, Widness JA. Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. J Perinatol 2005;25(1):21-25.
- Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics 2005;115:1299-306.
- Alves-Dunkerson JA, Hilsenrath PE, Cress GA, Widness JA. Cost analysis of a neonatal point-of-care monitor. Am J Clin Pathol 2002;117:809-18.
- Lin JC, Strauss RG, Kulhavy JC, Johnson KJ, Zimmerman MB, Cress GA, et al. Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics 2000;106(2):E19.
- Peake M, Mazzachi B, Fudge A, Bais R. Bilirubin measured on a blood gas analyser: a suitable alternative for near-patient assessment of neonatal jaundice? Ann Clin Biochem 2001;38:533-40.
- Papadea C, Foster J, Grant S, Ballard SA, Cate JC, Southgate WM, et al. Evaluation of the i-STAT portable clinical analyzer for point-of-care blood testing in the intensive care units of a university children's hospital. Annals of Clinical & Laboratory Science 2002;32:231-43.
- DeNicola LK, Kissoon N, Abram HSJ, Sullivan KJ, Delgado-Corcoran C, Taylor C. Noninvasive monitoring in the pediatric intensive care unit. Pediatric Clinics of North America 2001;48:573-88.
- Carter BG, Wiwczaruk D, Hochmann M, Osborne A, Henning R. Performance of transcutaneous PCO2 and pulse oximetry monitors in newborns and infants after cardiac surgery. Anaesth Intensive Care 2001;29:260-65.
- Hofman LF. Human saliva as a diagnostic specimen. J Nutr 2001;131:1621S-1625S.
- Holm-Hansen C, Tong G, Davis C, Abrams WR, Malamud D. Comparison of oral fluid collectors for use in a rapid point-of-care diagnostic device. Clinical and Diagnostic Laboratory Immunology 2004;11:909-12.
- Khalil OS. Non-invasive glucose measurement technologies: an update from 1999 to the dawn of the new millennium. Diabetes Technology & Therapeutics 2004;6:660-97.
- Siegemund M, van Bommel J, Ince C. Assessment of regional tissue oxygenation. Intensive Care Med 1999;25(10):1044-60.
- Kricka LJ. Microchips, microarrays, biochips and nanochips: personal laboratories for the 21st century. Clinica Chimica Acta 2001;307:219-23.
- Meier FA, Jones BA. Point-of-care testing error: sources and amplifiers, taxonomy, prevention strategies, and detection monitors. Arch Pathol Lab Med 2005;129:1262-67.
- Kost GJ, Ehrmeyer SS, Chernow B, Winkelman JW, Zaloga GP, Dellinger RP, et al. The laboratory-clinical interface. Point-of-care testing. Chest 1999;115:1140-54.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
TC monitoring in NICU - the value of tcpO2
Presented by Daniele De Luca (MD,PhD), Associate Professor of Neonatology, Chef de Service - Medical Director, Pediatrie et Reanimation Neonatale - Pediatrics and Neonatal Critical Care, GH Paris Sud - South Paris University Hospitals Watch the webinarScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars