Printed from acutecaretesting.org
May 2003
STAT testing in the future
STAT TESTING PROCESSES HAVE EVOLVED
Constant evolutions in laboratory processes have been necessary to meet the needs of its customers for rapid testing. The technology to perform such testing has also changed.
Developments in both instrumentation and laboratory computerization have enabled the laboratory to provide more timely information.
Today, internal laboratory processes are highly automated and efficient. With increased emphasis on acute treatment and outpatient care centers it becomes increasingly important to provide consistent, accurate STAT test results directly to the caregiver.
A four-part process
There are four main parts to the STAT testing process: orders, specimen collection, testing, and reporting of results.
Whereas orders were formerly handwritten by a physician and then transcribed into a computer by staff, direct physician entry of orders into a computerized system is becoming more and more common.
Specimen collection by a centralized phlebotomy department has evolved into more decentralized collection. Inside the laboratory, automated specimen handling (robotics) has changed the analytical process.
Result reporting has undergone changes. Results delivered electronically directly to the physician or caregiver are replacing printed reports. Such system-wide changes in all these processes lead us to examine the best way to perform STAT testing both inside and outside the laboratory.
In the early 1980s, the laboratory often developed two different, parallel processes for handling routine and STAT orders. Routine testing was generally conducted by clusters of analyzers in the laboratory performing a selected test or group of tests on a batch of specimens.
Because the methods employed involved long incubation times or frequent changes of reagents, analyzers required constant tending by a technologist. Time pressures led to a separate process pathway to be developed for STAT testing using dedicated analyzers or analyzers with limited testing menus.
These menus included electrolyte, glucose, creatinine, amylase, and bilirubin testing.
Initially, decentralized testing shortened TAT
In the 1990s, analyzers were moved from the laboratory to remote locations, providing STAT capabilities in the emergency department and other critical care settings.
These small labs eliminated the delay between specimen collection, test analysis, and patient treatment. Such labs were very expensive to operate since continuous staffing of a remote location required additional laboratory staff.
In addition, duplicate backup equipment had to be maintained in the lab in case of equipment failures. Nevertheless, testing on remote locations provided a net gain in turnaround time (TAT) by shortening the front-end processes of specimen delivery, centrifugation, distribution to analytical platforms, and reporting to the caregiver.
The 1990s also saw the introduction of analyzers that could identify and prioritize STAT testing, perform tests in a randomized fashion, and be interfaced with a laboratory information system (LIS).
That, together with the proven reliability of pneumatic tube systems, made it possible for remote STAT labs to be re-absorbed into the main lab. Because of these changes, focus shifted from STAT testing to STAT collection and delivery.
Two separate processes were developed for procuring specimens for STAT testing and routine testing. Requests for STAT collections became more numerous, until phlebotomy departments could not handle the workload.
That led to large increases in phlebotomy staff and utilization of other departments, including nursing personnel, to handle STAT collections.
Is STAT testing today different from routine?
Laboratory automation systems, capable of handling the entire testing process from specimen receipt to result reporting, are increasingly being installed in hospitals.
These automated systems have "leveled the playing field" in the laboratory by treating all specimens in the same fashion and forcing the testing process to follow only one pathway. It is not so much that a routine test has been elevated to STAT priority, but that the turnaround for routine testing has improved.
The automated system becomes the "owner" of the entire analysis from start to finish.
It dictates the exact circumstances for testing and controls the entire process - from the type of container in which the specimen must be collected, the exact placement of labels, centrifugation, de-capping, aliquoting and delivery of specimens to analyzers, and final storage.
Results are generated in a consistent manner no matter the priority, however recognition and response to truly critical requests have become more difficult to handle.
WHY NEAR PATIENT TESTING?
Emphasis on shorter hospital stays, more outpatient and acute patient care, and the development of outlying surgical centers have once again focused attention on the need for STAT testing.
Front-end processing and back-end reporting, such as specimen collection and result delivery, have two to three times the impact on TAT than the actual instrument analytical time.
A duality of STAT testing is developing - a "lab STAT", which is in essence a collection priority, and a life-critical STAT, which relates more to a location than to a particular patient. Patients that require a high level of care are frequently segregated within the hospital to quite specific locations.
Meeting STAT turnaround requirements for critical testing such as blood gases, electrolytes, etc. will focus on bringing the testing to locations such as inside a surgical suite, an Intensive Care Nursery, a remote day surgery center, or the emergency department.
Remote locations within or near a hospital, where transport of specimens to the main lab is inadequate and a very rapid turnaround is desirable, may be considered candidates for Near Patient Testing (NPT).
Testing in these departments may be seen as an "island of need" due to the distance from the laboratory and the need for a rapid TAT. These "islands of need" require a very rapid TAT, the ability to perform the analysis on small sample volumes (especially in applications involving pediatric/newborn or geriatric patients), instruments that are reliable but easy to operate, and results presented in an easy-to-read format.
Shifting a test from a centralized facility to a remote-location operation may increase the associated costs to the organization by 2-3 times.
Even though this type of testing carries with it a higher cost for testing materials, and nursing and laboratory labor, the cost to the hospital may be offset by better patient management and faster movement of patients from critical care beds to less expensive step-down units and floors.
It is this benefit to the patient care and hospital health that makes near patient testing practical and worthy of consideration.
THE NEAR PATIENT TESTING SOLUTION
Equipment used in NPT may have a larger test menu than a POC device and may increase its flexibility for use.
Training should basically enable staff to properly collect and introduce a specimen into the sample port of the analyzer and to accurately enter in patient demographic information. Expanding the operator base may cause difficulties in maintaining competency of all the operators.
Therefore, limitations as to who may operate analyzers may be called for.
NPT will drive a need to redefine responsibilities as well as processes. The role of the laboratory is in understanding and meeting all the regulatory requirements in the operations of the equipment and providing training and system oversight.
On the other hand, a strong POC organization will work to establish the necessary policy and procedures to oversee the day-to-day operations including: quality control review, instrument management, training non-laboratory operators, maintaining regulatory documentation, and addressing compliance issues.
Fundamental to the success of the operation of the system is a reliable and stable analyzer platform. The analytical platform must be operator friendly for use by non-laboratory staff and have the ability to remain unattended in a remote location for days.
Efficient handling of data from ordering to resulting is also crucial. When practical, test results should be reviewed in the lab prior to release to the LIS/HIS network and, in many cases, prior to being used for clinical decisions.
OUT OF THE AREA, BUT STILL IN CONTROL
In the past few years, the concept of decentralized "Near Patient Testing" with a centralized "Point Of Control" in the laboratory has become increasingly popular.
Remote support of analyzers, ownership of the measurement and data handling processes, and responsibility for the test result quality remain with the laboratory even though some aspects of testing have been delegated to the decentralized staff.
With such a placement of the analyzers comes a redefinition of "lab". Each analyzer location is a "point of testing" and, in essence, a minilab. Specimens are introduced into the analyzer by non-laboratory staff but all aspects of instrument performance and acceptability of test results remains with the lab staff.
Interpreting the results generated by the analyzer is entrusted to only trained laboratory personnel. Integration of the instruments through the HIS/LIS systems allows timely review of results with return of the result electronically to a preselected location.
The data may be returned to the area where the instrument is located or routed directly to the physician through the use of clinical management systems.
No longer does the caregiver need to wait for the delivery of printed reports before making clinical decisions. This can be a giant step toward more rapid and pertinent patient care. The process flows electronically from the physician orders to results with minimal staff intervention.
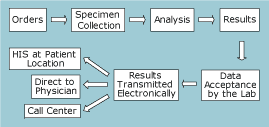
FIG. 1
DATA MANAGEMENT
The key to success for future NPT systems will be the adoption of a common connectivity standard. Connecting different devices to one software platform will allow the laboratory to cluster instruments in an NPT area while not requiring multiple data management systems. The optimum number of data management systems in near patient testing is two. One system would be used for managing point-of-care glucose testing, and a second for all other testing platforms. Additional data management systems would introduce unwanted complexity, require additional funding and consume valuable space in areas generally quite crowded where these systems typically reside.
New data acceptance processes utilize a "spreadsheet" presentation of data from multiple analyzers with color coding of unusual results instead of the traditional "roll and scroll" approach to viewing data. These data acceptance modules also offer auto-acceptance of "test" results that meet established criteria. The ability also exists to build customized rules for special handling such as recollects, comments or other special handling. It is this integration of electronic assistance within the process that is germane to the success of an NPT system.
THE OPERATORS
Maintaining a pool of staff that can collect and introduce specimens into the analyzer in a consistent manner is vital for the success of any NPT system. There are two types of operator in an NPT system.
The first type is the laboratory technologist and the second type is the trained non-technical staff whose task is to introduce the specimen into the analyzer. The monitoring of analyzer functions, quality control as well as accessing the results generated, will always be the responsibility of the laboratory.
To ensure reliable patient test results, review by qualified medical technologists is essential. The technologist review will include test results that will cross traditional "department" lines.
Data review will be the responsibility of highly trained technical staff that are comfortable reviewing results from multiple platforms and that are available every day on each shift. The POC department may or may not be staffed to fulfill such a responsibility necessitating defined, reliable backup from other sources (e.g. the central laboratory).
The data management system should maintain all records related to staff training and competency examinations. The staff introducing a specimen into the analyzer for analysis may include nurses, perfusionists, respiratory therapy technologists, and other unit staff.
A thorough training program must be designed to train them to become proficient operators. They should not be made into lab technologists, but they need to have an adequate understanding of the tasks they are expected to perform. It will be necessary to establish levels of competency for these operators. This includes the means of testing their competency at prescribed intervals.
The key to a successful operator program is to limit testing to a select group of people, to get consistent, high-quality results.
THE ANALYZERS
Analyzers commonly used inside a laboratory area are generally not suitable for placement in outlying locations. Proper lockouts and controls need to be in place to restrict use of the equipment to trained staff.
Otherwise, if the analyzer is out there, someone, trained or not, will most likely use it. It is necessary that all operators and technologists understand and appreciate how the analyzer works and what results they are actually getting.
Improperly collected specimens that are run by untrained staff can have a large negative impact on the quality of the results obtained and lead to inadequate or incorrect diagnosis and treatment. Therefore access control to the analyzer is important in a near-patient-testing environment.
Using passwords to restrict access to the analyzer is essential in maintaining adequate control over the process. Passwords should be customizable to allow access to perform only those tasks for which users have been trained.
For example, a nurse may be allowed to introduce specimens only, a technologist may have access to quality control, maintenance, and reagent functions, while a system manager may have access to the setup functions and other analyzer definitions.
Vendors have begun building additional functionality into their equipment. Such instrument control systems allow access to diagnostic routines, quality control systems, and the ability to monitor and control the equipment remotely. The instrument must have the ability to detect and warn of certain important error conditions.
When a condition has been detected that will affect the results generated, the equipment should cease to operate and display that an error condition exists. It is especially critical that analyzers in remote locations alert the laboratory to these conditions.
Equipment operations must be straightforward and require limited maintenance visits, as frequent maintenance visits and reagent refills are impractical. Analyzers may be placed in locations quite remote from the laboratory or impractical to access (e.g. entering operating rooms during surgical procedures).
Equipment that is robust enough to withstand the rigors of remote operations while providing a consistent, high-quality result will make NPT a vital link in patient diagnosis and treatment.
Technology and equipment are only a part of the support that a vendor must provide to meet the needs of a near patient system. Companies will need to develop high-level support in three areas: instrument servicing, data management system support, and connectivity support to the laboratory information system (LIS). Generally, vendors do an adequate job in the first two areas, but have traditionally drawn the line when it comes to LIS support.
Companies that provide a liaison between their system and the LIS recognize that the successful completion of the testing process does not stop until results are in the hands of the caregiver. As these NPT systems move from a traditional LIS-analyzer connection to one using an instrument-generated order and testing approach, their involvement in this area will be increasingly vital.
When evaluating new equipment, hospital IT departments are becoming an essential part of the evaluation team. The evaluation of the connectivity aspect and the vendor's ability to provide LIS support has been recognized by hospitals as vital to the success of any near-patent-testing system.
The choice of an analyzer by evaluating technical performance may actually be secondary to the performance and support concerning these connectivity issues.
Future near-patient analytical systems will require strong technical support from the vendor in partnership with the POC team, IT and the LIS vendors.
RESULT VERIFICATION
The laboratory technologist verifying test results from an NPT analyzer will have no concern whether the test was physically performed within the laboratory or at some remote location. The next generation of laboratory data management will not focus on where the data is generated, but on how it will be properly reviewed.
A two-tiered structure to STAT "laboratory" testing is developing: work performed in the general lab and work performed "Near Patient".
We need to be moving to a single system for data review. There should be no difference in the review of completed results electronically transmitted to the lab for evaluation and results generated within the laboratory.
Laboratories have traditionally been concerned with testing performed within their walls. Laboratory testing, no matter where it is performed, is still part of the laboratory. It is important to handle all data with identical scrutiny.
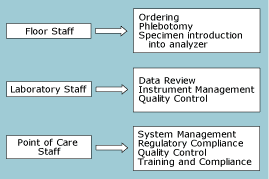
FIG. 2
Much will need to be done to break down barriers to efficiency between lab staff and those from other hospital departments that have become testing partners but located externally to the traditional laboratory.
STAT testing, when performed using whole blood on equipment within a reasonably close proximity to the patient and reported via the LIS/HIS, will result in the fast turnaround desired at one of these special locations.
Cooperative partnerships
We have to go further than just simply moving analytical equipment. The nursing, physicians and unit staff, are in a cooperative partnership with laboratory staff. It is this formation of cooperative partnerships that will enable the NPT system to be successful and enable better patient management.
We can improve operations of the entire hospital's patient management system by using analytical systems placed remote to a central hub. No longer is the sole responsibility of the entire analysis process handled by systems internal to the laboratory.
The ultimate goal is to deliver high-quality test values to critical patients, to those in remote locations, distant from the central laboratory in a timely manner.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








