Printed from acutecaretesting.org
December 1997
The roles of transcutaneous carbon dioxide measurement in a respiratory support center
VENTILATORY FAILURE
Definitions
If an individual has an arterial oxygen tension (pO2(a)) which is less than 8 kPa (60 mmHg), he/she is said to be in respiratory failure.
In Type I respiratory failure the arterial carbon dioxide tension (pCO2(a)) is normal or low.
In Type II respiratory failure (or ventilatory failure) the pCO2(a) is abnormally elevated, that is, it is greater than 6.5 kPa (48.8 mmHg).
The level of pCO2(a) is inversely proportional to the level of ventilation. Thus, for example, if ventilation is halved the pCO2(a) doubles. The causes of ventilatory failure include an inadequate ventilatory drive, weak respiratory muscles or an excessive respiratory impedance due to, for example, a stiff chest wall in scoliosis or a high resistance to air flow in chronic bronchitis.
Effects of hypercapnia
In normal healthy subjects an elevated pCO2(a) is a powerful stimulus to increase ventilation. Respiratory drive can be tested using protocols where the subject rebreathes from a bag. The pCO2(a) rises approximately linearly and so does ventilatory effort.
In clinical situations, as hypercapnia develops the patient fails to increase the level of ventilation appropriately and the stimulus is therefore ineffective. The excess of CO2 leads to a build-up of hydrogen ions, that is, a respiratory acidosis develops which may adversly affect cardiac function.
Other effects of the elevated pCO2(a) include a flapping tremor and vasodilatation which causes headaches and may contribute to ankle swelling. If the pCO2(a) rises high enough the patient will lapse into coma.
If pCO2(a) is chronically elevated (for > 24 hours) the acidosis becomes compensated as the kidneys generate extra bicarbonate to buffer the hydrogen ions. The increase in buffering capacity especially in the cerebrospinal fluid reduces the sensitivity to further changes in pCO2(a). A chronically elevated pCO2(a) therefore leads to a reduction in ventilatory drive.
Limitations of pulse oximetry in the assessment of ventilation
Pulse oximetry is a simple method for the measurement of arterial oxygen levels, expressed as the saturation sO2(p). It is widely used as a surrogate measure of ventilation.
However while the level of oxygen in arterial blood is related to ventilation, the relationship between ventilation and sO2(p) is not linear. This is because of the sigmoidal shape of the oxyhemoglobin dissociation curve.
In the higher ranges of pO2(a) large changes in value are accompanied by relatively small changes in sO2(p). Once the pO2(a) falls below 8 kPa (60 mmHg), the sO2(p) falls much more quickly and is a more sensitive index of change in pO2(a).
If a patient with normal lungs and gas transfer develops hypoventilation, then large changes can occur in pO2(a) and pCO2(a) with little change in sO2(p), as shown in the example below:
Example
Starting gas tensions:
pCO2(a) 5 kPa (37.5 mmHg),
pO2(a) 14 kPa (105 mmHg),
sO2(p) approx. 98 %
Ventilation halved:
pCO2(a) 10 kPa (75 mmHg),
pO2(a) 9kPa (67.5 mmHg),
sO2(p) approx. 92 %
A pO2(a) of 10 kPa (75 mmHg) may be sufficient to cause coma (CO2 narcosis), but the sO2(p) is misleadingly normal. If additional oxygen is given to a patient, then the sO2(p) no longer gives any useful information about the level of ventilation.
RESPIRATORY SUPPORT TECNIQUES
Central to each of the respiratory support techniques described below is the optimisation of the arterial blood gases in patients who have compromised ventilatory function and/or gas exchange. In each, it is important to remember that hypoxia and hypercapnia are to be avoided. Below is a brief history of the techniques and a discussion of the evidence of their benefits.
Long-term domiciliary noninvasive ventilation
Mechanical devices to assist ventilation were described as long as 150 years ago [1]. The first devices were external negative pressure ventilators (ENPV) such as the 'iron lung' or 'tank' ventilator.
Development of the technique was closely linked to the poliomyelitis epidemics which affected large numbers in Europe and the USA in the early part of this century [2]. However, positive pressure ventilation using a tracheostomy tube improved survival in acute poliomyelitis and soon replaced ENPV as the ventilation method of choice [3,4].
Some patients with poliomyelitis did not recover to full ventilatory independence and domiciliary ventilation services were developed for their care.
Tracheostomy ventilation can be effective for long-term home care [5], but it has important disadvantages, including the risk of occlusion by secretions as well as impaired coughing, swallowing and speech [6,7]. ENPV also has disadvantages for home ventilation [8,9] Noninvasive intermittent positive pressure ventilation (NiIPPV) has been shown to have advantages over ENPV and tracheostomy ventilation for domiciliary treatment and is now the method of choice.
NiIPPV was described as early as 1912 in conjunction with anesthesia but the equipment required the constant presence of an attendant to hold the mask, and a pressurized gas source, making it unsuitable for home use [10] Despite improved ventilator pumps the mask seal remained a major difficulty until the development of modern silicon-based rubber (Fig. 1).
The first use of home NiIPPV was described only as recently as 1984 [11]. A detailed study of the physiological effects of the technique was published in 1987 [12]. This showed improvements in overnight gas exchange and in daytime blood gas tensions even though the ventilator was only used at night.
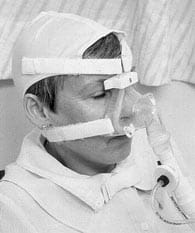 |
FIG. 1: A patient using nasal
intermittent positive pressure ventilation.
|
NiIPPV is most commonly used for the long-term domiciliary support of patients with chronic ventilatory failure. The mask is usually only worn during sleep when self-ventilation is least effective (Fig. 2).
There is evidence of a benefit in a number of conditions leading to chronic ventilatory failure. These include neuromuscular disorders such as the muscular dystrophies and myopathies [12], chest wall deformities including scoliosis and kyphosis [13,14], and previous tuberculosis [15].
Chronic obstructive pulmonary disease (COPD) may also lead to chronic ventilatory failure, and some patients have been treated with long term NiIPPV, but it is not as effective as in those disorders where the pulmonary deficit is restrictive [16].
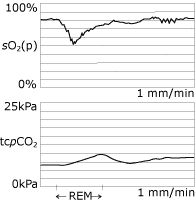 |
FIG. 2: Ventilatory failure is
usually more pronounced during sleep.
|
The benefits of long-term home NiIPPV include an improvement in the symptoms of ventilatory failure, such as breathlessness, fatigue and poor quality sleep. The number of days spent hospitalized can be reduced [17] and patients previously confined at home may return to work or school.
Many studies have shown that daytime blood gas tensions are improved by nocturnal ventilation [12-20]. Three large series have reported the long-term survival with NiIPPV[17,21,22].
Overall, these show that survival is longest in patients with a stable chest wall or neuromuscular condition and less certain in those with a progressive neuromuscular disorder, COPD or bronchiectasis. In one series, the mean survival for patients with a scoliosis was eight years compared with three years for those with COPD [22].
Noninvasive ventilation for acute ventilatory failure
NiIPPV is being increasingly used for the treatment of acute on chronic ventilatory failure with the aim of avoiding intubation or supporting patients for whom invasive ventilation is not thought to be appropriate.
Most of the published data describing the use of NiIPPV for acute ventilatory failure are from patients with COPD. This treatment is not only used in Respiratory Support Centers, but also in intensive care units (ICUs) and general medical wards though most reports of its use have been published by specialist centers.
Although there are several series describing the use of NiIPPV for acute ventilatory failure in patients with COPD, there is still controversy over its effectiveness. It appears that only a sub-group of patients, which is difficult to identify, benefit.
In uncontrolled series it has been shown that NiIPPV may rapidly improve arterial blood gas tensions (ABGs) [23,24] especially if the initial pCO2(a) is elevated, but this has not been a consistent finding [25,26].
The effect of NiIPPV on the frequency of intubation has been examined in comparison to historical controls [27,28] and in randomized controlled trials [29,30]. NiIPPV can, it seems, reduce the frequency of intubation, though in none of the studies has a statistically significant survival benefit been demonstrated [31].
Weaning from invasive ventilation
One motivation for the use of NiIPPV in acute ventilatory failure is to avoid intubating patients who may subsequently prove difficult to wean from invasive ventilation.
Most patients who are intubated and ventilated have this done electively to facilitate a surgical procedure. They have good respiratory function and after a short time most can be extubated without difficulty.
Patients with long-term ventilatory disability who are intubated during an acute deterioration may prove more difficult to wean and can remain ventilator-dependent for months or even years.
When this occurs NiIPPV may be a useful tool in reestablishing ventilator independence, and where this is not completely achieved, in supporting patients in the long term, allowing discharge to home.
The survival of patients to discharge from hospital after nonelective invasive ventilation in the ICU is only around 40 to 50 % [32-34]. Many patients fail to wean from invasive ventilation and between 6 and 16 % die on a general ward after discharge from the ICU [35].
One cause of these deaths is probably recurrent ventilatory failure. There is evidence to suggest that the outcome of patients invasively ventilated in the ICU can be improved if they are referred to a Respiratory Support Center.
Descriptive studies have shown that NiIPPV can be useful in patients with long-term ventilator dependence in ICU [36,37]. There have been no controlled trials of the use of NiIPPV for weaning, but when it is used as part of a comprehensive care program survival may be improved compared to historical controls [38].
In our experience, survival to discharge from hospital for patients with long-term ventilator dependence is around 90 %. Although 60 % of patients require some form of ventilatory support at discharge, this is usually only used at night.
Factors other than noninvasive ventilation are important in improving the likelihood of survival and weaning from ventilation. Diagnostic review may reveal a treatable cause for ventilatory failure or allow inappropriate treatment to be stopped.
Asthma appears to be overdiagnosed and the corticosteroids used to treat it can cause a loss of muscle strength, immunosupression, sleep disruption and sometimes psychosis. Resetting the day/night cycle, which is difficult to maintain in the ICU allows sedative medication to be withdrawn and improves respiratory drive.
Patients on long-term ventilation are often allowed to retain CO2, and as described above this reduces respiratory drive. The ABGs should therefore be normalized before weaning is attempted.
Long-term oxygen therapy
Chronic obstructive pulmonary disease such as bronchitis and emphysema is most commonly caused by cigarette smoking. Cessation of smoking is to be strongly encouraged if the progression of the disease is to be slowed.
In patients who become hypoxic due to lung damage survival is poor. The adverse effects of chronic hypoxia include confusion and poor concentration, pulmonary hypertension, right heart strain, arrhythmias and peripheral oedema. Long-term oxygen therapy (LTOT) to correct the hypoxia has been shown to improve survival.
The first controlled study to demonstrate this was the NOTT study in 1980, which showed that continuous oxygen gave a greater survival benefit than oxygen therapy used only at night [39].
Patients were given a fixed rate of oxygen, 1 L/min in the day and 2 L/min at night. Subsequently, the MRC study in 1981 compared treatment with oxygen for 15 hours a day with no treatment, and showed a survival benefit intermediate between that of nocturnal and continuous oxygen in the NOTT study [40].
In the MRC study the oxygen flow rate was a minimum of 2 L/min and increased when necessary to keep the sO2(p) > 90 %. In this and subsequent studies an elevated pCO2(a) was a predictor of a poor outcome.
It is well known that patients with COPD may develop hypercapnia if given additional oxygen for hypoxia (Fig. 3), although there is debate about the mechanism [41].
It seems likely that in the NOTT, MRC and subsequent studies, the prescription of LTOT at fixed rather than individualized flow rates led in to an elevated pCO2(a), which may have adversly affected the outcome.
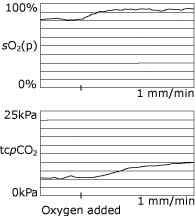 |
FIG. 3: Oxygen therapy may
produce hypercapnia
|
THE METHOD OF tcpCO2 MEASUREMENT
Principles of the electrode system
The transcutaneous electrode comprises a heating element, temperature sensor, silver/silver chloride reference electrode, and an H+ measuring electrode. Heating the skin leads to local vasodilatation and increased skin permeability to CO2.
The released CO2 from the skin diffuses through the selectively permeable membrane of the electrode, reacting with the electrolyte between the membrane and electrode. This reaction forms carbonic acid which dissociates into H+ and HCO-3.The potential difference is proportional to [H+] and thus CO2 concentration.
Factors affecting measured tcpCO2
Electrode site and electrode temperature are the main determinants of quality in transcutaneous measurements. The electrode site should allow optimal coupling of the electrode to well perfused skin with minimal movement.
A site over a joint or a large muscle is likely to result in shifting of the electrode and should be avoided. Although the electrode is small, flat skin contours are preferable. For long measuring periods, the electrode and its lead should be secured to the skin to avoid distortion caused by the weight of the lead pulling the electrode from the skin.
These factors and the need to select a capillary bed under lean tissue - free of obvious veins, tattoos, excessive hair or regular exposure to the aging affects of the sun - make the inside of the forearm nearest to the monitor the optimal site in most patients. Compression of the capillary bed underneath the measuring electrode caused by taping or bandaging over the electrode, should be avoided.
The site temperature must be high enough to promote gas diffusion through the skin without causing tissue damage. The increase in temperature increases metabolism in the skin and CO2 production. tcpCO2 is therefore higher than pCO2(a).
The difference is generally small, being on the order of 1 kPa (7.5 mmHg), but the higher the temperature selected the greater the disparity. A site temperature of 42.5 Celcius is suitable for overnight measurements on a single site in adults.
Adults will not normally develop skin burns at this setting. If the skin at the optimal site appears particularly translucent or sensitive (for example in elderly patients or those on long-term steroid treatment), the electrode temperature should be reduced to 42.0 Celcius and the site changed every 4 hours.
ELECTRODE AND SITE PREPARATION
In regular daily use the electrode and electrode membranes remain moist between measurements. However, storage of the electrode in electrolyte or re-membraning prior to measurement will improve the quality of measurements.
Conditioning of the electrode can be achieved by removing the electrode membrane and soaking the electrode for hours in electrolyte solution. Gentle cleaning of the electrode with the disk papers is necessary prior to remembraning the electrode. Single point gas calibration is necessary before use or if the measurement site is changed.
Measurement site selection is detailed above. Excess hair should be removed and the site cleaned thoroughly with an alcohol wipe. The site is dried with tissue paper and a fixation ring applied. Additional tape can be used to secure the adhesive ring but this should not cause compression to the underlying capillary bed.
After filling the electrode cup within the fixation ring with three drops of contact solution the electrode should be twisted into the cup, ensuring that the electrode lead lies towards the monitor, or if the forearm is selected, downwards towards the wrist.
The electrode lead can be looped and then taped to the skin before a gauze bandage is wrapped lightly over the electrode and secured over the electrode lead. Once stable values are measured, a quick check for patient movement distortion should be made.
The electrode should be relocated if the CO2 measurement changes with 0.3 kPa (2.25 mmHg).
CLINICAL PROTOCOLS INCLUDING tcpCO2 MEASUREMENTS
Initiation of noninvasive ventilation
The initial symptoms of ventilatory failure include lethargy, breathlessness on exertion, early morning headache and poor sleep quality. Examination may reveal a cause for ventilatory failure such as diaphragmatic weakness, and signs of end organ dysfunction, such as right ventricular heave and ankle oedema.
Laboratory investigations can show polycythaemia and right heart strain on the electrocardiogram. In most cases the daytime ABGs will be abnormal with an elevated pCO2(a) and a low pO2(a), but in some symptomatic patients the daytime ABGs may be normal.
Overnight studies will most often reveal far greater abnormality as respiratory impedance rises and ventilatory drive falls during sleep [43].
Overnight studies should include a minimum of pulse oximetry and tcpCO2 measurement. tcpCO2 measurement is particularly important in patients with no lung disease (e.g. those with a chest wall or neuromuscular disorder), since in these patients the sO2(p) may be relatively normal despite a greatly raised pCO2(a) (see chapter 1 above and Fig. 4).
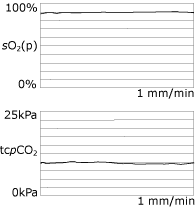 |
FIG. 4: An elevated
tcpCO2 with a misleadingly
normal sO2(p)
|
In patients using additional oxygen the sO2(p) is of no value as a surrogate measure of ventilation. In this circumstance it is best if the overnight study can be performed without added oxygen, but it is mandatory to measure tcpCO2 if it cannot.
Once ventilatory failure has been diagnosed and the decision made to commence noninvasive ventilation, the treatment must be titrated to correct for hypoventilation.
The ventilators used for NiIPPV are either volume or pressure preset. The tidal volume delivered to the lungs of an intubated patient can be guaranteed (with a volume preset ventilator) but during NiIPPV the volume varies since leaks from the mask vary considerably [17].
The only way to be sure that the patient is adequately ventilated at night is to monitor tcpCO2. Pulse oximetry may be helpful, especially if it is markedly abnormal before treatment, but as explained above, it is not a good surrogate measure of ventilation in patients with normal lungs, and is of no use in patients who are receiving additional oxygen.
In our practice the ventilator is initially set up with the patient awake, and according to his or her comfort. The preset volume or pressure is subsequently titrated against continuous overnight tcpCO2 measurements (Fig. 5).
We would consider a range of 4 to 7 kPa (30 - 52.5 mmHg) to be acceptable values of tcpCO2. It is important not to reduce the pCO2(a) too far as this may lead to problems with upper airway occlusion [43] and dangerous, prolonged apneas if the patient is disconnected from the ventilator.
If the nocturnal tcpCO2 is acceptable but the sO2(p) remains < 90 % additional oxygen may be prescribed. When the daytime ABGs are normalized and the patients are confident using the equipment, they are allowed home.
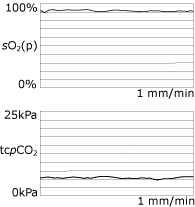 |
FIG. 5: Normalized overnight
monitoring when using NiIPPV
|
On subsequent visits the overnight studies are repeated to ensure that the prescription of ventilation remains optimal. Long term surveillance is important, as in many patients ventilatory failure is due to a progressive disorder and the requirement for ventilatory support may change over time [44].
Noninvasive ventilatory support in acute ventilatory failure
If NiIPPV is introduced during an acute episode of ventilatory failure, a similar approach is used but continuous monitoring to allow timely decisions is even more important. To determine whether the treatment is working it is necessary to chart the CO2.
Arterial measurements cannot be made continuously, and if additional oxygen is used this will invalidate sO2(p) as a surrogate measure of ventilation. If the tcpCO2 does not fall with NiIPPV, the patient may require intubation and ventilation.
Weaning from invasive ventilation
Whenever changes are made to ventilatory support it is important to check the effect on the ABGs. This is especially important during weaning, when ventilatory support is being reduced.
Sleep deprivation impairs respiratory drive, and sedative medication to maintain sleep at night will also affect this. Re-establishing day/night cycles is important in the optimization of respiratory function before weaning is attempted in patients who are chronically ventilator-dependent.
Using noninvasive measurements rather than repeated arterial stabs or in-dwelling lines will contribute to improved sleep at night.
At the time of extubation changes in blood chemistry may occur quickly, and continuous monitoring with sO2(p) and tcpCO2 will give an early indication if the patient’s own ventilatory effort is inadequate. As in other circumstances, the sO2(p) will be falsely reassuring if additional oxygen is being used.
If the tcpCO2 rises by more than 1.5 to 2 kPa (11.3 - 15 mmHg) after extubation, the initiation of NiIPPV may be considered. Patients with a tracheostomy tube may practice with NiIPPV before extubation, with the tube capped and the cuff deflated.
In our experience, around 60 % of patients who are difficult to wean from invasive ventilation require some form of noninvasive support after extubation.
Titrating long-term oxygen therapy
LTOT is most frequently prescribed for patients with COPD. The precise recommendations for the use of LTOT vary among countries but they are governed by the same general principles. It is important to ensure that the prescription is appropriate since it is an expensive treatment, and while it may prolong life it does not necessarily improve the quality of life.
It may indeed reduce the quality of life since it restricts the patient’s mobility. The patient should have hypoxia of a sufficient severity to warrant treatment. It must be stable and not secondary to an infective or other exacerbation of airways disease.
In the UK, this level of hypoxia is taken as a pO2(a) < 7.3 kPa (54.8 mmHg) alone or a pO2(a) < 8 kPa (60 mmHg) in the presence of evidence of end organ dysfunction, including right heart strain on electrocardiogram, peripheral oedema or polycythaemia.
Once the patient has been identified as likely to benefit from LTOT, the oxygen level should be titrated up to the minimum flow rate which corrects the hypoxia. In our unit we perform a titration study during daytime wakefulness, recording sO2(p) and tcpCO2 continuously.
The oxygen flow rate is increased in 0.25 to 0.5 L/min increments at hourly intervals. When it seems that the optimal oxygen flow rate has been found (sO2(p) > 90 %, tcpCO2 < 8 kPa (60 mmHg)), ABGs are checked again to ensure that hypoxia is corrected and that hypercapnia has not been induced.
Different flow rates of oxygen may be required by day and by night, so the study is repeated overnight. Using this method, around 90 % of patients are prescribed oxygen flow rates of < 1 L/min.
In our experience, despite the careful titration of LTOT prescription around 10 % of patients develop hypercapnia which is symptomatic or which we consider puts them at risk of rapid deterioration when they are given additional oxygen. We have started these patients on NiIPPV. In 50 %, nocturnal ventilation improves the daytime gases sufficiently to make LTOT unnecessary.
In the other half, most use NiIPPV at night and LTOT during the day only. In previous studies looking at LTOT an elevated pCO2(a) has been associated with a worse outcome.
We have shown that in patients unable to tolerate LTOT who are started on NiIPPV the level of pCO2(a) is no longer a predictor of outcome suggesting that adequate treatment of ventilatory failure improves survival [45].
SUMMARY
The range of techniques available to support patients with respiratory impairment has grown considerably in the last two decades and among the most important have been NiIPPV and LTOT.
In order to establish which patients are likely to benefit from NiIPPV as a long-term treatment in the home overnight, CO2 estimations are required and the best available method to obtain these is using tcpCO2 measurements.
Matching the patient’s requirements with the ventilator’s output also requires the measurement of tcpCO2 during undisturbed sleep.
When short-term treatment for ventilatory failure with NiIPPV is introduced, progress can be most usefully judged by calibrated tcpCO2 recording. This allows limits to treatment to be set, dictating when it may be safely withdrawn or when it can be judged to have failed and more invasive methods of support considered.
When weaning patients from invasive ventilation, the effects of changes in ventilation can be best appreciated by continuous monitoring. This can avoid crash reintubation and will indicate when NiIPPV may be needed in order to bridge the gap between full ventilator dependence and full self-ventilation.
Long-term oxygen therapy may induce hypercapnia. This should be excluded at the initiation of treatment, not only on daytime arterial blood gas tensions but also during sleep when ventilation is most vulnerable.
This is again best achieved using tcpCO2. The tendency to use sO2(p) as a surrogate measure of ventilation should be resisted where possible, as it may lead to dangerous hypercapnia being overlooked.
In particular where additional oxygen is prescribed, repeated ABGs should be taken and ideally tcpCO2 will be recorded continuously by day and night. If the tcpCO2 is elevated, then NiIPPV may be a more appropriate treatment.
References+ View more
- Dalziel J. On sleep and an apparatus for promoting artificial respiration. Br Assoc Adv Sci 1938; 2: 127-28.
- Shambaugh GEJ, Harrison WGJ, Farrell JI. The treatment of respiratory paralysis of poliomyelitis in a respiratory chamber. JAMA 1930; 94: 1371-73.
- Bower AG, Bennett VA, Dillon JB, Axelrod B. Investigation on the care and treatment of poliomyelitis patients. Ann West Med Surg 1950; 4: 686-716.
- Lassen HCA. A preliminary report on the 1952 epidemic of poliomyelitis in Copenhagen with special reference to the treatment of acute respiratory insufficiency. Lancet 1953; 1: 37-41.
- Hoeppner VH, Cockcroft DW, Dosman JA, Cotton DJ. Nightime ventilation improves respiratory failure in secondary kyphoscoliosis. Am Rev Respir Dis 1984; 129: 240-43.
- Buckwalter JA, Sasaki CT. Effect of tracheotomy on laryngeal function. Otolaryngeal Clinics of North America 1984; 17, 1: 41-48.
- Bach JR. Alternative methods of ventilatory support for the patient with ventilatory failure due to spinal cord injury. J Am Paraplegia Soc 1991; 14: 158-74.
- Hill NS. Clinical applications of body ventilators. Chest 1986; 90, 6: 897-905.
- Levy RD, Bradley TD, Newman SL, Macklem PT, Martin JG. Negative pressure ventilation: Effects on ventilation during sleep in normal subjects. Chest 1989; 95,1: 95-99.
- Bunnell S. The use of nitrous oxid and oxygen to maintain anesthesia and positive pressure for thoracic surgery. JAMA 1912; LVIII: 835-39.
- Delaubier A. Traitement de l’insuffisance respiratoire chronique dans les dystrophies muscularies. In: Anonymous memoires de certificat d’etudes superieures de reeducation et redaption functionelles. Paris: Universite R Descarte, 1984: 1-124.
- Ellis ER, Bye PTP, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with a neuromuscular disease. Am Rev Respir Dis 1987; 135: 148-52.
- Ellis ER, Grunstein RR, Chan S, Bye PTP, Sullivan CE. Noninvasive ventilatory support during sleep improves respiratory failure in kyphoscoliosis. Chest 1988; 94: 811-15.
- Smith IE, Laroche CM, Jamieson SA, Shneerson JM. Kyphosis secondary to tuberculosis osteomyelitis as a cause of ventilatory failure. Clinical features, mechanisms and management. Chest 1996; 110: 1105-10.
- Jackson M, Smith I, King M, Shneerson J. Long term non-invasive domiciliary assisted ventilation for respiratory failure following thoracoplasty. Thorax; 49: 915-19.
- Simonds AK, Elliott MW. Outcome of domiciliary nasal intermittent positive pressure ventilation in restrictive and obstructive disorders. Thorax 1995; 50: 604-09.
- Bach JR, Alba A. Management of chronic alveolar hypoventilation by nasal ventilation. Chest 1990; 97: 52-57.
- Kerby GR, Mayer LS, Pingleton SK. Nocturnal positive pressure ventilation via nasal mask. Am Rev Respir Dis 1987; 135: 738-740.
- Carroll N, Branthwaite MA. Control of nocturnal hypoventilation by nasal intermittent positive pressure ventilation. Thorax 1988; 48: 349-53.
- Ludwigs UG, Baehrendtz S, Wanecek M, Matell G. Mechanical ventilation in medical and neurological diseases: 11 years experience. J Int Med 1991; 229: 117-24.
- Leger P, Bedicam JM, Cornette A, Reybat-Degat O, Langevin B, Polu JM, Jeanni L, and Robert D. Nasal intermittent positive pressure ventilation. Long term follow-up in patients with severe chronic respiratory insufficiency. Chest 1994; 105: 100-05.
- Chailleux E, Fauroux B, Binet F, Dautzenberg B, Polu JM. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. Chest 1996; 109: 741-49.
- Meduri GU, Conoscenti CC, Menashe P, Nair S. Noninvasive face mask ventilation in patients with acute respiratory failure. Chest 1989; 95: 865-70.
- Elliott MW, Steven MH, Phillips GD, Branthwaite MA. Non-invasive mechanical ventilation for acute respiratory failure. BMJ 1990; 300: 358-60.
- Conway JH, Hitchcock RC, Godfrey RC, Carroll MP. Nasal intermittent positive pressure ventilation in acute exacerbations of chronic obstructive pulmonary disease - a preliminary study. Respir Med 1993; 87: 387-94.
- Meecham-Jones DJ, Paul EA, Graham-Clarke C, Wedzicha JA. Nasal ventilation in acute exacerbations of chronic obstructive pulmonary disease: effect of ventilator mode on arterial blood gas tensions. Thorax 1994; 49: 1222-24.
- Brochard L, Isabey D, Piquet J, Amaro P, Mancebo J, Messadi A, Brun-Buisson C, Rauss A, Lemaire F, Harf A. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med 1990; 323: 1523-30.
- Vitacca M, Rubini F, Foglio K, Scalvini S, Nava S, Ambrosino N. Non-invasive modalities of positive pressure ventilation improve the outcome of acute exacerbations in COLD patients. Int Care Med 1993; 19: 450-55.
- Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomised, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 1995; 151: 1799-806.
- Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simmoneau G, Benito S, Gasparetto A, Lemaire F, Isabey D, Harf A. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333: 817-22.
- Bott J, Carroll MP, Conway JH, Keilty SEJ, Ward EM, Brown AM, Paul EA, Elliott MW, Godfrey RC, Wedzicha JA, Moxham J. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet 1993; 341: 1555-57.
- Gracey DR, Naessens JM, Krishnan I, Marsh HM. Hospital and post hospital survival in patients mechanically ventilated for more than 29 days. Chest 1992; 101: 211-14.
- Stauffer JL, Fayter NA, Graves B, Cromb M, Lynch JC, Goebel P. Survival following mechanical ventilation for acute respiratory failure in adult men. Chest 1993;104: 1222-29.
- Swinburne AJ, Fedullo AJ, Bixby K, Lee DK, Wahl GW. Respiratory failure in the elderly: analysis of outcome after treatment with mechanical ventilation. Arch Intern Med 1993; 153: 1657-62.
- Bion J. Outcomes in intensive care. BMJ 1993; 307: 953-54.
- Udwadia ZF, Santis GK, Steven MH, Simonds AK. Nasal ventilation to facilitate weaning in patients with chronic respiratory insufficiency. Thorax 1992; 47: 715-18.
- Restrick LJ, Scott AD, Ward EM, Feneck RO, Cornwell WE, Wedzicha JA. Nasal intermittent positive-pressure ventilation in weaning intubated patients with chronic respiratory disease from assisted intermittent, positive-pressure ventilation. Respir Med 1993; 87: 199-204.
- Smith IE, Shneerson JM. A progressive care programme for prolonged ventilatory failure: analysis of outcome. Br J Anaes 1995; 75: 399-404.
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. Ann Intern Med 1980; 93: 391-98.
- Report of the Medical Research Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet; 1: 681-85.
- Hanson CW, Marshall BE, Frasch FH, Marshall C. Causes of hypercarbia with oxygen therapy in patients with chronic obstructive pulmonary disease. Crit Care Med 1996; 24: 23-28.
- Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis 1982; 126: 758-62.
- Delguste P, Aubert-Tulkens G, Rodenstein DO. Upper airway obstruction during nasal intermittent positive airway pressure hyperventilation during sleep. Lancet 1991; 338: 1295-97.
- Smith IE, Shneerson JM. Secondary failure of nasal intermittent positive pressure ventilation using the Monnal D: effects of changing ventilator. Thorax 1997; 52: 89-91.
- Sivasothy P, Smith IE, Shneerson JM. Mask intermittent positive pressure ventilation in chronic hypercapnic respiratory failure due to chronic obstructive pulmonary disease. Eur Respir J 1998; 11: 34-40.
References
- Dalziel J. On sleep and an apparatus for promoting artificial respiration. Br Assoc Adv Sci 1938; 2: 127-28.
- Shambaugh GEJ, Harrison WGJ, Farrell JI. The treatment of respiratory paralysis of poliomyelitis in a respiratory chamber. JAMA 1930; 94: 1371-73.
- Bower AG, Bennett VA, Dillon JB, Axelrod B. Investigation on the care and treatment of poliomyelitis patients. Ann West Med Surg 1950; 4: 686-716.
- Lassen HCA. A preliminary report on the 1952 epidemic of poliomyelitis in Copenhagen with special reference to the treatment of acute respiratory insufficiency. Lancet 1953; 1: 37-41.
- Hoeppner VH, Cockcroft DW, Dosman JA, Cotton DJ. Nightime ventilation improves respiratory failure in secondary kyphoscoliosis. Am Rev Respir Dis 1984; 129: 240-43.
- Buckwalter JA, Sasaki CT. Effect of tracheotomy on laryngeal function. Otolaryngeal Clinics of North America 1984; 17, 1: 41-48.
- Bach JR. Alternative methods of ventilatory support for the patient with ventilatory failure due to spinal cord injury. J Am Paraplegia Soc 1991; 14: 158-74.
- Hill NS. Clinical applications of body ventilators. Chest 1986; 90, 6: 897-905.
- Levy RD, Bradley TD, Newman SL, Macklem PT, Martin JG. Negative pressure ventilation: Effects on ventilation during sleep in normal subjects. Chest 1989; 95,1: 95-99.
- Bunnell S. The use of nitrous oxid and oxygen to maintain anesthesia and positive pressure for thoracic surgery. JAMA 1912; LVIII: 835-39.
- Delaubier A. Traitement de l’insuffisance respiratoire chronique dans les dystrophies muscularies. In: Anonymous memoires de certificat d’etudes superieures de reeducation et redaption functionelles. Paris: Universite R Descarte, 1984: 1-124.
- Ellis ER, Bye PTP, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with a neuromuscular disease. Am Rev Respir Dis 1987; 135: 148-52.
- Ellis ER, Grunstein RR, Chan S, Bye PTP, Sullivan CE. Noninvasive ventilatory support during sleep improves respiratory failure in kyphoscoliosis. Chest 1988; 94: 811-15.
- Smith IE, Laroche CM, Jamieson SA, Shneerson JM. Kyphosis secondary to tuberculosis osteomyelitis as a cause of ventilatory failure. Clinical features, mechanisms and management. Chest 1996; 110: 1105-10.
- Jackson M, Smith I, King M, Shneerson J. Long term non-invasive domiciliary assisted ventilation for respiratory failure following thoracoplasty. Thorax; 49: 915-19.
- Simonds AK, Elliott MW. Outcome of domiciliary nasal intermittent positive pressure ventilation in restrictive and obstructive disorders. Thorax 1995; 50: 604-09.
- Bach JR, Alba A. Management of chronic alveolar hypoventilation by nasal ventilation. Chest 1990; 97: 52-57.
- Kerby GR, Mayer LS, Pingleton SK. Nocturnal positive pressure ventilation via nasal mask. Am Rev Respir Dis 1987; 135: 738-740.
- Carroll N, Branthwaite MA. Control of nocturnal hypoventilation by nasal intermittent positive pressure ventilation. Thorax 1988; 48: 349-53.
- Ludwigs UG, Baehrendtz S, Wanecek M, Matell G. Mechanical ventilation in medical and neurological diseases: 11 years experience. J Int Med 1991; 229: 117-24.
- Leger P, Bedicam JM, Cornette A, Reybat-Degat O, Langevin B, Polu JM, Jeanni L, and Robert D. Nasal intermittent positive pressure ventilation. Long term follow-up in patients with severe chronic respiratory insufficiency. Chest 1994; 105: 100-05.
- Chailleux E, Fauroux B, Binet F, Dautzenberg B, Polu JM. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. Chest 1996; 109: 741-49.
- Meduri GU, Conoscenti CC, Menashe P, Nair S. Noninvasive face mask ventilation in patients with acute respiratory failure. Chest 1989; 95: 865-70.
- Elliott MW, Steven MH, Phillips GD, Branthwaite MA. Non-invasive mechanical ventilation for acute respiratory failure. BMJ 1990; 300: 358-60.
- Conway JH, Hitchcock RC, Godfrey RC, Carroll MP. Nasal intermittent positive pressure ventilation in acute exacerbations of chronic obstructive pulmonary disease - a preliminary study. Respir Med 1993; 87: 387-94.
- Meecham-Jones DJ, Paul EA, Graham-Clarke C, Wedzicha JA. Nasal ventilation in acute exacerbations of chronic obstructive pulmonary disease: effect of ventilator mode on arterial blood gas tensions. Thorax 1994; 49: 1222-24.
- Brochard L, Isabey D, Piquet J, Amaro P, Mancebo J, Messadi A, Brun-Buisson C, Rauss A, Lemaire F, Harf A. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med 1990; 323: 1523-30.
- Vitacca M, Rubini F, Foglio K, Scalvini S, Nava S, Ambrosino N. Non-invasive modalities of positive pressure ventilation improve the outcome of acute exacerbations in COLD patients. Int Care Med 1993; 19: 450-55.
- Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomised, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 1995; 151: 1799-806.
- Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simmoneau G, Benito S, Gasparetto A, Lemaire F, Isabey D, Harf A. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333: 817-22.
- Bott J, Carroll MP, Conway JH, Keilty SEJ, Ward EM, Brown AM, Paul EA, Elliott MW, Godfrey RC, Wedzicha JA, Moxham J. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet 1993; 341: 1555-57.
- Gracey DR, Naessens JM, Krishnan I, Marsh HM. Hospital and post hospital survival in patients mechanically ventilated for more than 29 days. Chest 1992; 101: 211-14.
- Stauffer JL, Fayter NA, Graves B, Cromb M, Lynch JC, Goebel P. Survival following mechanical ventilation for acute respiratory failure in adult men. Chest 1993;104: 1222-29.
- Swinburne AJ, Fedullo AJ, Bixby K, Lee DK, Wahl GW. Respiratory failure in the elderly: analysis of outcome after treatment with mechanical ventilation. Arch Intern Med 1993; 153: 1657-62.
- Bion J. Outcomes in intensive care. BMJ 1993; 307: 953-54.
- Udwadia ZF, Santis GK, Steven MH, Simonds AK. Nasal ventilation to facilitate weaning in patients with chronic respiratory insufficiency. Thorax 1992; 47: 715-18.
- Restrick LJ, Scott AD, Ward EM, Feneck RO, Cornwell WE, Wedzicha JA. Nasal intermittent positive-pressure ventilation in weaning intubated patients with chronic respiratory disease from assisted intermittent, positive-pressure ventilation. Respir Med 1993; 87: 199-204.
- Smith IE, Shneerson JM. A progressive care programme for prolonged ventilatory failure: analysis of outcome. Br J Anaes 1995; 75: 399-404.
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. Ann Intern Med 1980; 93: 391-98.
- Report of the Medical Research Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet; 1: 681-85.
- Hanson CW, Marshall BE, Frasch FH, Marshall C. Causes of hypercarbia with oxygen therapy in patients with chronic obstructive pulmonary disease. Crit Care Med 1996; 24: 23-28.
- Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis 1982; 126: 758-62.
- Delguste P, Aubert-Tulkens G, Rodenstein DO. Upper airway obstruction during nasal intermittent positive airway pressure hyperventilation during sleep. Lancet 1991; 338: 1295-97.
- Smith IE, Shneerson JM. Secondary failure of nasal intermittent positive pressure ventilation using the Monnal D: effects of changing ventilator. Thorax 1997; 52: 89-91.
- Sivasothy P, Smith IE, Shneerson JM. Mask intermittent positive pressure ventilation in chronic hypercapnic respiratory failure due to chronic obstructive pulmonary disease. Eur Respir J 1998; 11: 34-40.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Role of transcutaneous CO2 monitoring in high risk respiratory patients
Presented by Gil De Oliveira, MD
Watch the webinarRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









