Printed from acutecaretesting.org
January 2004
What is EQA
Introduction
Over the years the American term proficiency testing (PT) has to some extent become synonymous with the European term of External Quality Assessment (EQA) and most laboratories have regarded the difference in terminology as one of the many vagaries of American English.
However, a recent document published by the International Federation of Clinical Chemistry (IFCC), Education and Management Division, Committee of Analytical Quality challenges this statement. The Guidelines for the Requirement for the Competence of EQAP organizers clearly differentiates between the two [1].
The voluntary guidelines are directed to External Quality Assurance Program (EQAP) organizers to demonstrate competence by formal compliance with internationally acceptable requirements.
The guide is essentially based on ISO 43-1: 1997; ISO EN/IEC 17025: 1999; ISO 9000: 1994; ILAC G13: 2000; and CPA(EQA) [2-6]. A new terminology has emerged from the document of EQAP.
Definitions
Proficiency Testing (PT) is generally accepted as the term used in North America. The main focus of PT is essentially on the performance evaluations for regulatory purposes. Successful participation for laboratory accreditation is required by several US institutions such as the Centers for Medicare & Medicaid Services (CMS), the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the College of American Pathologists (CAP).To pass proficiency testing, clinical laboratories must meet desirable standards laid down under the Clinical Laboratory Improvement Act (CLIA) [7].
Due to sanctions, PT schemes have to respect current analytical performance and therefore have wide acceptability limits. The fact that a laboratory may lose their license if they do not pass PT may influence the use of special practices for EQA samples [8].
A major limitation of the PT scheme is the tendency to maintain the quality at a certain level and they are unable to stimulate improvement of quality above this level [9,10].
External Quality Assessment Scheme (EQAS) is the general term used in Europe and South America.
EQAS focuses also on performance evaluations but the purpose of the scheme is educational. With the exception of Germany, there is no link between the results obtained in Western Europe and the condition of the reimbursement of tests. EQA will only reflect routine quality if all users are allowed to treat the EQA samples in the same way as patient samples, even with the risk of making a mistake.
Since there are no sanctions, more stringent acceptability limits can be used and should produce a more realistic reflection of quality of lab performance.
Acceptance limits based on biological variation and clinical decision limits can therefore be used to promote awareness of analytical deficiencies and stimulate method improvement. Interference studies and specificity studies are often undertaken in these schemes.
In Europe there is an increase in the number of schemes that are emphasizing quality improvement through well-designed educational schemes and focus is on Quality Assurance rather than Assessment. The IFCC have therefore added another terminology to differentiate these schemes from the traditional EQAS.
External Quality Assurance Program (EQAP) is an interlaboratory comparison designed and operated to assure one or more of the following aspects, which will be described in the following sections:
- Participant performance evaluation
- Interpretation (including advice to the clinician on laboratory requests and diagnosis).
- Method performance evaluation.
- Postmarketing vigilance of in vitro diagnostic devices [11]
- Continuous education, training and help.
The primary intention of the activities of an EQAP in laboratory medicine shall be to support quality improvement of the services provided by the participating laboratories for the benefit of the patient.
Participant performance evaluation
This is essentially covered by both PT and EQA and is an evaluation of a participant’s measuring performance compared with other laboratories using the same methods. International and regional schemes can be used to assess one or more of the following [12]:- The quality of the analytical performance of the participant’s laboratory
- The state of the art of participating laboratories
- Intralaboratory variation
- Interlaboratory variation
- Relationship between calibration procedures and analytical results
- Relationship between analytical procedures and results
- Relationship between commercial reagents and results
- Relationship between analytical instruments and results
- State-of-the-art values for concentration of the analytes
- Systematic deviations for the individual laboratory from state-of-the-art values or reference target values
A well-designed EQA Scheme should provide information on most of the ten points.
Interpretation
Examples of Clinical Interpretation Schemes include UKNEQAS Clinical Cases, UKNEQAS Toxicology, WEQAS Porphyrin and WEQAS Macroprolactin.In the WEQAS Porphyrin Scheme the analytical and interpretative elements are examined. The following is an example of the clinical information distributed together with an EQA sample and the interpretation, as it should look according to an expert advisor:
A blind sample is distributed along with clinical information, e.g. a urine sample with a total porphyrin concentration of 1400 nmol/L was distributed along with the following clinical information: “46-yr-old gentleman complaining of skin fragility and blistering lesions on the backs of his hands.”
The correct interpretation is given by an expert advisor and is made available to all participants along with a summary of the responses. Key phrases used by the expert (in bold in the example) are used to evaluate the responses.
Expert report commentRaised urinary porphyrin requires further investigation. Please send faeces (approx. 5 g) and EDTA blood.The raised urinary porphyrin is very suggestive that one of the cutaneous porphyrias is responsible for the patient’s skin lesions (which are typical). Confirmation of this and identification of the type of porphyria requires fractionation of the individual porphyrins in urine and stool and examination of plasma by fluorescence emission spectroscopy. This will probably entail referral to a specialist laboratory. All three specimens should be sent. It is particularly important to differentiate between cutaneous porphyrias in which potentially fatal acute attacks may occur (variegate porphyria and hereditary coproporphyria) and those in which they do not (porphyria cutanea tarda and congenital erythropoietic porphyria). Furthermore effective treatments exist for porphyria cutanea tarda but not the other types. |
Summary of results45 out of the 73 laboratories, (62 %) would have carried out the appropriate further investigation on all three samples, whilst 27 % would have carried out selective analysis of either blood urine or feces, eight would have also carried out Urine PBG on the sample. Three would have referred the sample, two laboratories would have investigated for family history and two for alcohol abuse. |
Method performance evaluation
Laboratories are recommended to use in vitro diagnostic devices (IVDs) according to the manufacturer’s instructions. This evaluation requires the use of sample material as close as possible to patient samples. By comparing a homogeneous group of users of the same IVD the method performance can be evaluated [13]. This type of evaluation has proved to be particularly difficult in blood gas analysis due to the inherent instability of the patient sample. Over the last few years the WEQAS Blood Gas Scheme conducted studies to assess the performance of routine blood gas analyzers in the UK using a tonometered whole-blood material [14].The use of material as close as possible to the patient sample minimizes any matrix effect and allows the assessment of accuracy.
By using dedicated designs and samples, EQA can be used for studying robustness of methods, sensitivity to interferences, linearity, recovery, specificity and also pre- and postanalytical factors. Examples are found in UKNEQAS Steroid Hormones, WEQAS Mainline Chemistry, and WEQAS Lipid Scheme.
The WEQAS Lipid Scheme recently carried out an interference study to investigate the effect of a turbid, lipemic (chylomicron) sample on the performance of the major analyzers for lipid analysis.
MaterialThe sample was a serum pool prepared from six fresh blood donations. All six samples appeared grossly lipemic and on standing had a significant chylomicron layer. The donations were pooled, gentamicin added, mixed well and aliquotted immediately. Unlike the other WEQAS pools, this pool was not filtered as this can decrease the triglyceride concentration. The aliquots were stored at –70 ºC until dispatch. Results
Preanalytical effects such as inadequate mixing of sample, variable time of analysis resulting in separation of chylomicron layer, and analyzer sampling technique can also be tested when dedicated EQA samples are designed. |
Postmarket vigilance of IVD Directive (prEN 14136)
EQAS is able to contribute to postmarketing monitoring of IVD MD as mentioned in Directive 98/79/EC on IVD MD [15] to the benefit of both manufacturers and users. A well-designed scheme can provide data of comparison between new or established analytical procedures; demonstrate transferability of procedures; and disclose deficiencies of the IVD over long-term use. The use of repeat samples can demonstrate reproducibility and the effect of changes in the property of the IVD.An important function of EQAS is to provide evidence of trueness of results through the use of appropriate samples analyzed by reference methods and to identify possible deficiencies of the IVD.
Where EQAS data indicate an apparent problem, the situation should be investigated further.
Performance should be evaluated to a method of higher metrological order and on appropriate clinical samples to limit matrix effects.
In perceived malfunction, EQA organizers should work closely with the manufacturer and, if appropriate, contact the competent authorities.
Continuous education, training and help
EQA Schemes play an important role in the continuous education of laboratory staff. The “non-profit”-making schemes in the UK, WEQAS and UKNEQAS have played key roles in Regional and National Audit, promoted harmonization of results, e.g. HbA1c standardization.
WEQAS hosts annual scientific meetings covering topical, clinical and scientific issues, whilst UKNEQAS is coorganizer of the annual FOCUS meetings of the Association of Clinical Biochemists. WEQAS organizes training days on EQA interpretation and problem solving and supplements this with educational material via its website www.weqas.com.
The development of interactive web-based EQA Schemes provide data and information that would have been impossible to access on a conventional paper EQA Report. Experience in WEQAS has shown that the powerful database query gives participants a wealth of information on method and analyzer performance and is an invaluable troubleshooting aid. Analytical errors associated with batches of reagent or calibrator changes can be identified quickly.
It is anticipated that the readily available educational material will provide a better understanding and improvement in interpretation of data with a long-term outcome of improved analytical quality.
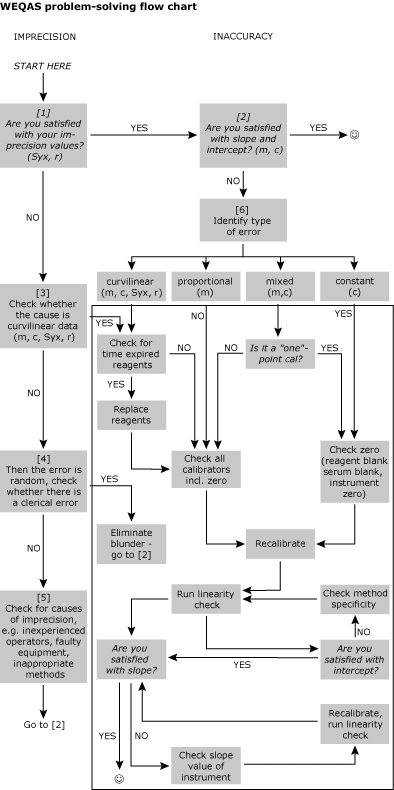
Conclusion
No, EQA is not just another word for proficiency testing.
EQA in medical laboratories have evolved over the past 50 years to provide more sophisticated systems compared with the simple participants’ analytical performance evaluation of earlier years.
Many already meet the requirements of the new EQAP definition. Pre- and postanalytical elements have been included in EQA Scheme design, introducing elements of quality assurance rather than assessment.
EQA must provide a key role in the assessment of methods and in their postmarketing vigilance. Educational elements of the scheme are now pivotal in most national EQA Schemes and can be further improved by appropriate scheme designs according to clear objectives.
Schemes that do not include Quality Assurance as defined in EQAP will ultimately fail to improve quality, and laboratories should consider these elements in their choice of EQA provider.
The key objective of EQA is continuous quality improvement within laboratory medicine and EQA providers should therefore include quality improvement of scheme design as an essential requirement of the service. It is hoped that accreditation of EQA Schemes should facilitate this improvement.
References+ View more
- Maziotta D, Harel D, Schumann G, Libeer J-C. Guidelines for the requirement for the competence of EQAP organizers in medical laboratories. IFCC/EMD/C-AQ, 2003.
- Requirements for the competence of providers of proficiency testing schemes, ILAC G13, 1998.
- ISO EN/IEC 17025: 1999.
- ISO 9000: 1994.
- Standards for EQA Schemes in Laboratory Medicine. CPA, 2002.
- Proficiency testing by interlaboratory comparisons – Part 1: development and operation of proficiency testing schemes. ISO/IEC Guide 43: 1998.
- Clinical Laboratory Improvement Amendments (CLIA) of 1988, US Public Law 100-575.
- Cembrowski GS, Vanderline RE. Survey of special practices associated with College of American Pathologists proficiency testing in the Commonwealth of Pennsylvania. Arch Pathol Lab Med 1988; 112: 374-77.
- Libeer J-C. Role of external quality assurance schemes in assessing and improving quality in medical laboratories. Clin Chim Acta 2001; 309: 173-77.
- Libeer J-C, Baadenhuijsen H, Fraser C, Hyltoft Petersen P, Ricos C, Stöckl D, Thienpoint L. Characterization and classification of external quality assessment schemes (EQA) according to objectives such as evaluation of method and participant bias and standard deviation. Eur J Clin Chem Clin Biochem 1996; 34: 665-78.
- Use of external quality assessment schemes in the assessment of the performance of in vitro diagnostic products. prEN 14136, 2001.
- Aronsson T, Bjørnstad P, Johansson SG, Leskinen E, Raabo E, De Verdier C-H. Interlaboratory quality control with investigation of different methodological characteristics. Scan J Clin Lab Invest 1978; 38: 53-62.
- Stöckl D, Libeer J-C, Reinhauer H, Thienpont LM, De Leenheer AP. Accuracy-based assessment of proficiency testing results using serum from single donations: possibilities and limitations. Clin Chem 1996; 42: 469-70.
- Thomas MA. Performance of blood gas analysis – the UK experience. Ann Biol Clin 2003; 61: 213-18.
- Directive 98/79/EC of the European Parliament and the Council of 27 October 1998 on in vitro diagnostic medical devices. Official Journal of the European Communities 7.12.98, L331/1-37.
References
- Maziotta D, Harel D, Schumann G, Libeer J-C. Guidelines for the requirement for the competence of EQAP organizers in medical laboratories. IFCC/EMD/C-AQ, 2003.
- Requirements for the competence of providers of proficiency testing schemes, ILAC G13, 1998.
- ISO EN/IEC 17025: 1999.
- ISO 9000: 1994.
- Standards for EQA Schemes in Laboratory Medicine. CPA, 2002.
- Proficiency testing by interlaboratory comparisons – Part 1: development and operation of proficiency testing schemes. ISO/IEC Guide 43: 1998.
- Clinical Laboratory Improvement Amendments (CLIA) of 1988, US Public Law 100-575.
- Cembrowski GS, Vanderline RE. Survey of special practices associated with College of American Pathologists proficiency testing in the Commonwealth of Pennsylvania. Arch Pathol Lab Med 1988; 112: 374-77.
- Libeer J-C. Role of external quality assurance schemes in assessing and improving quality in medical laboratories. Clin Chim Acta 2001; 309: 173-77.
- Libeer J-C, Baadenhuijsen H, Fraser C, Hyltoft Petersen P, Ricos C, Stöckl D, Thienpoint L. Characterization and classification of external quality assessment schemes (EQA) according to objectives such as evaluation of method and participant bias and standard deviation. Eur J Clin Chem Clin Biochem 1996; 34: 665-78.
- Use of external quality assessment schemes in the assessment of the performance of in vitro diagnostic products. prEN 14136, 2001.
- Aronsson T, Bjørnstad P, Johansson SG, Leskinen E, Raabo E, De Verdier C-H. Interlaboratory quality control with investigation of different methodological characteristics. Scan J Clin Lab Invest 1978; 38: 53-62.
- Stöckl D, Libeer J-C, Reinhauer H, Thienpont LM, De Leenheer AP. Accuracy-based assessment of proficiency testing results using serum from single donations: possibilities and limitations. Clin Chem 1996; 42: 469-70.
- Thomas MA. Performance of blood gas analysis – the UK experience. Ann Biol Clin 2003; 61: 213-18.
- Directive 98/79/EC of the European Parliament and the Council of 27 October 1998 on in vitro diagnostic medical devices. Official Journal of the European Communities 7.12.98, L331/1-37.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars







