Printed from acutecaretesting.org
July 2003
All about base excess – to BE or not to BE
Background
The acid-base status in the human organism is described in terms of arterial pH, arterial pCO2 and the extracellular base excess, Base(ecf).
Base excess of extracellular fluid is defined as the titratable base of extracellular fluid.
Base excess in blood, cBase(B), which was defined before cBase(ecf), is also in use, and this is the titratable base of whole blood.
Both are titrated to pH = 7.40 and pCO2 = 40 mmHg (5.3 kPa) at T = 37.0 °C (98.6 °F).
For clinical use I recommend the use of cBase(ecf).
Base excess of extracellular fluid is a quantity that reflects only the non-respiratory (metabolic) component of acid-base disturbances. It is the most used "non-respiratory" quantity for the diagnosis of acid-base disturbances and is calculated and presented by all blood gas analyzers produced today worldwide.
Names, definitions and abbreviations
The following table gives an overview of the terminology and definitions used in this article and in other literature.
|
Name |
Abbreviation |
Definition |
|
Buffer Base |
BB |
Sum of concentration of bicarbonate, albumin and hemoglobin |
|
Delta Buffer Base |
ΔBB |
The change in BB from 'normal' |
|
Base Excess in blood or Actual Base Excess |
cBase(B) or ABE |
Actual Base Excess, the concentration of titratable base when the blood is titrated with strong base or acid to a plasma pH of 7.4 at pCO2 of 40 mmHg (5.3 kPa) and 37 °C (98.6 °F) at the actual saturation. Positive values (base excess) indicate a relative deficit of non-carbonic acids; negative values (base deficit) indicate a relative excess of non-carbonic acids |
|
Base Excess in extracellular fluid or Standard Base Excess or Base Excess in vivo |
cBase(ecf) or SBE |
Standard Base Excess is an in vivo expression of base excess. It refers to a model of the extracellular fluid (the blood volume is diluted with the interstitial fluid) and is calculated using a standard value for the hemoglobin concentration (5 g/dL or 3 mmol/L) of the total extracellular fluid. |
When base excess is used (here and in other literature) it may denote both base excess in blood and base excess in extracellular fluid.
History
In the first half of the 20th century, when diagnosing acid-base disturbances blood was analyzed by extraction to determine total CO2 content, ctCO2(B). This measurement and pH were the acid-base parameters. It was difficult with these two values to distinguish between metabolic and respiratory acid-base disturbances.
In 1948, Singer and Hastings introduced the concept of buffer base. Blood buffer base is practically the sum of bicarbonate, albuminate and hemoglobinate, which is about 48 mmol/L in normal subjects.
Singer and Hastings soon recommended the use of delta buffer base (ΔBB) as a measure of metabolic acid-base disturbance. ΔBB was the change in buffer base from the normal buffer base of pH = 7.40, pCO2 = 5.33 kPa (40 mmHg) at 37.0 °C (98.6 °F). This was the first proposal of base excess!
In the beginning of the 1950s, Astrup developed his equilibration technique in connection with the patients with poliomyelitis and paralysis of the respiratory muscles. Astrup's aim was to get an indicator of how much one should artificially ventilate such patients, and therefore it was the pCO2 that was most important for him.
Based on this, in 1960 Siggaard-Andersen and coworkers introduced an acid-base nomogram for the calculation of all relevant acid-base values of blood.
The nomogram was called the Siggaard-Andersen curve nomogram (Fig. 1). It used a pH-logpCO2 coordinate system. On the nomogram there were two curves: the buffer base curve and the base excess curve.
It was now possible to read buffer base and base excess directly if the pH-logpCO2 line was determined. To draw the line one had to equilibrate blood with two different gases with a known CO2 content and measure pH in the two equilibrated blood samples. The pCO2 had to be calculated from the actual barometric pressure and the CO2 content (Vol %) of the equilibration gas.
The construction of the first base excess curve on the Siggaard-Andersen curve nomogram had been done by many equilibration procedures. Base excess was defined as equal to zero when pH = 7.40, pCO2 = 5.33 kPa (40 mmHg) at 37 °C (98.6 °F) for all concentrations of hemoglobin! Base excess could later be calculated in computers without using a nomogram.
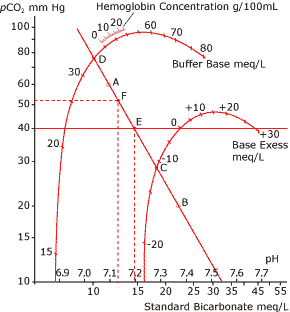
FIG. 1.
A: pH value measured after equilibration at
pCO2 = 60 mmHg
B: pH value measured after equilibration at
pCO2 = 20 mmHg
The pH/logpCO2 is drawn as a straight
line through A and B
C: Base excess
D: Buffer base
E: Standard Bicarbonate
F: Actual pH and pCO2
Base excess in blood versus base excess in extracellular fluid
In the beginning of the 1960s, a controversy about base excess arose.
The criticism came in particular from Schwartz and Relman in Boston (the Boston school). The main reason for the criticism was that there was a difference between in vitro and in vivo titration with CO2.
One consequence of this was that base excess changed with changes in pCO2. When pCO2 is varied in vivo by CO2 inhalation or hyperventilation, not only blood but the whole extracellular fluid is equilibrated with the new pCO2. When pCO2 increases, pH tends to decrease more in the poorly buffered interstitial fluid than in the well-buffered blood. Hydrogen ions therefore tend to diffuse from the interstitial fluid to the blood, and this is registered as a fall in base excess of blood.
Siggaard-Andersen and coworkers now modified the computation of base excess and used as model the blood volume diluted with the interstital fluid. This model lowered the normal hemoglobin concentration to about 5 g/dL or 3 mmol/L, and this was decided to be used in the computation of cBase(ecf). This is the effective extracellular-fluid hemoglobin concentration.
Anemia has little effect on buffering the extracellular fluid and can therefore be neglected. The computation was now much more simple with only use of pH and cHCO3- (actual bicarbonate) and was then the most relevant measure of the metabolic disturbance in the acid-base status.
This new parameter was called base excess in the extracellular fluid (cBase(ecf)), but was also called in vivo base excess or standard base excess (SBE).
The great transatlantic acid-base debate
Schwartz and Relman continued to advocate for use of actual bicarbonate concentration instead of base excess, and in 1965 Bunker introduced the expression "the great transatlantic acid-base debate".
Since Severinghaus in San Francisco was one of the strongest advocates of extracellular base excess, the discussion was also named "the great transamerican acid-base debate"! In 1977, Severinghaus suggested a "detente" and offered a modified Siggaard-Andersen nomogram containing an estimate of the compensation of hypercapnia according to the Boston school's work.
The two schools remained unreconciled.
The criticism of the Boston school's way of interpreting
acid-base disturbances focused on the fact that by that method it
is necessary to learn six bicarbonate equations and rules and solve
them in one's head without paper and pencil!
In an American textbook from 1999 written by Martin, he explains
why he does not use base excess at all in blood gas interpretation.
The main reason is: "The negative terminology is confusing,
particularly when BE is negative and one hears the term negative
base excess".
I was a little astonished when I read this.
Calculation of base excess by blood gas analyzers
In 1980, I did a presentation for IFCC's Expert Panel for pH and Blood Gases and showed how the different blood gas analyzers calculated base excess of blood (cBase(B)).
The algorithms were very different. They all used pH, pCO2 and hemoglobin concentration. One company used pO2 in addition. Especially in the calculations of high values of base excess (values > 15 mmol/L, metabolic alkalosis), the difference in the calculations of base excess was up to 4-5 mmol/L, so it could have clinical consequences.
I invited the industry and the IFCC Expert Panel to work for more standardization in the calculations of base excess.
Twenty years later I repeated the investigation, and the standardization was much better than 20 years before. There were only small differences in the calculations. The calculations of cBase(ecf) are very standardized. The most used algorithm is:
cBase(ecf) = 16.2 × (pH - 7.40) - 24.8 + cHCO3-. Some manufacturers use 25 instead of 24.8; that means a change in base excess of 0.2 mmol/L.
Effect of oxygen saturation
One question that needs to be addressed is how much does variation of oxygen saturation of hemoglobin (sO2) influence the base excess result. When the blood passes the lungs and the sO2 is increased, for example from 70 % to 100 %, how much does this influence the base excess of blood? Oxyhemoglobin is a stronger acid than deoxyhemoglobin . The formula for calculating this is:
cBase(B,oxygenated) = cBase(B,actual) - 0.2 × ctHb × (1 - sO2)
when ctHb is in g/dL. 0.3 is used instead of 0.2 if the hemoglobin concentration is given in mmol/L. If we use a total hemoglobin concentration of 15 g/dL, the change will be -0.9 mmol/L, i.e. blood base excess is changed a little in an acidotic direction when the blood is oxygenated.
In earlier times (in the 1960s-1970s), when the original Astrup technique (equilibration method) was used as routine, it was common to do this correction for base excess.
At that time, all the calculations of the acid-base parameters were done manually and with help of the Siggaard-Andersen curve nomogram. The base excess was corrected from 100 % oxygenated blood to the actual saturation. The reason was that the equilibration of the blood was done with a high O2 content in the equilibration gas (more than 90 Vol % of oxygen).
Conclusion
Today, the discussion about base excess is still characterized a little by the transatlantic acid-base debate.
The main argument against base excess is the difference between in vivo and in vitro CO2 titration curves. Some authors call standard bicarbonate and base excess obsolete parameters.
It is wrong to talk about standard bicarbonate and base excess together.
Today, standard bicarbonate is an anachronism since the original Astrup technique is no longer in routine use. Base excess (extracellular fluid) is still, in my point of view, the key parameter for diagnosing non-respiratory (metabolic) disturbances in the acid-base status.
References+ View more
- Astrup P, Severinghaus JW. The history of blood gases, acids and bases. Munksgaard: Copenhagen, 1985.
- Kofstad J. Calculations of base excess on four different blood gas analyzing instruments (IL613, Corning175, Radiometer ABL2 and AVL940). In: Proceedings from IFCC's Workshop for the Expert Panel for pH and Blood Gases. Copenhagen: Private Press, 1980: 83-89.
- Kofstad J. Base Excess. A historical review - has the calculation of base excess been more standardised the last 20 years? Clin Chim Acta 2001; 307: 193-95.
- Martin L. All you really need to know to interpret arterial blood gases. 2nd ed. Lippincott, Williams & Wilkins, 1999.
- Peters JP, Van Slyke DD. Quantitative clinical chemistry, volume II, methods. Bailliere, Tindall and Cox, 1932.
- Schlichtig R, Grogono AW, Severinghaus JW. Current status of acid-base quantification in physiology and medicine. Respiration in Anesthesia, pathophysiology and clinical update.1998; 16,1: 211-33.
- Schwartz WB, Relman AS. A critique of the parameters used in evaluation of acid-base disorders. New Eng J Med 1963; 268: 1382-88.
- Severinghaus JW. Acid-base nomogram - a Boston-Copenhagen detente. Anest 1976; 45: 539-41.
- Severinghaus JW. Siggaard-Andersen and the "Great trans-Atlantic acid-base debate". Scand J Clin Lab Invest 1993; 214, Suppl 53: 99-104.
- Siggaard-Andersen O, Engel K. A new acid-base nomogram. An improved method for the calculation of the relevant blood acid-base data. Scand J Clin Lab Invest 1960; 12: 177.
- Siggaard-Andersen O. The Acid-Base Status of the Blood, 4th revised ed. Copenhagen: Munksgaard, 1974
- Siggaard-Andersen O. The Van Slyke equation. Scand J Clin Lab Invest 1977; 146, Suppl 37: 15-20.
- Siggaard-Andersen, Goethgen IH. Oxygen and acid-base parameters of arterial and mixed venous blood, relevant versus redundant. Acta Anaest Scand 1995; 39, Suppl 107: 123-28.
- Singer RB, Hastings AB. An improved clinical method for the estimation of disturbances of the acid-base balance of human blood. Medicine (Baltimore) 1948; 27: 223-42.
References
- Astrup P, Severinghaus JW. The history of blood gases, acids and bases. Munksgaard: Copenhagen, 1985.
- Kofstad J. Calculations of base excess on four different blood gas analyzing instruments (IL613, Corning175, Radiometer ABL2 and AVL940). In: Proceedings from IFCC's Workshop for the Expert Panel for pH and Blood Gases. Copenhagen: Private Press, 1980: 83-89.
- Kofstad J. Base Excess. A historical review - has the calculation of base excess been more standardised the last 20 years? Clin Chim Acta 2001; 307: 193-95.
- Martin L. All you really need to know to interpret arterial blood gases. 2nd ed. Lippincott, Williams & Wilkins, 1999.
- Peters JP, Van Slyke DD. Quantitative clinical chemistry, volume II, methods. Bailliere, Tindall and Cox, 1932.
- Schlichtig R, Grogono AW, Severinghaus JW. Current status of acid-base quantification in physiology and medicine. Respiration in Anesthesia, pathophysiology and clinical update.1998; 16,1: 211-33.
- Schwartz WB, Relman AS. A critique of the parameters used in evaluation of acid-base disorders. New Eng J Med 1963; 268: 1382-88.
- Severinghaus JW. Acid-base nomogram - a Boston-Copenhagen detente. Anest 1976; 45: 539-41.
- Severinghaus JW. Siggaard-Andersen and the "Great trans-Atlantic acid-base debate". Scand J Clin Lab Invest 1993; 214, Suppl 53: 99-104.
- Siggaard-Andersen O, Engel K. A new acid-base nomogram. An improved method for the calculation of the relevant blood acid-base data. Scand J Clin Lab Invest 1960; 12: 177.
- Siggaard-Andersen O. The Acid-Base Status of the Blood, 4th revised ed. Copenhagen: Munksgaard, 1974
- Siggaard-Andersen O. The Van Slyke equation. Scand J Clin Lab Invest 1977; 146, Suppl 37: 15-20.
- Siggaard-Andersen, Goethgen IH. Oxygen and acid-base parameters of arterial and mixed venous blood, relevant versus redundant. Acta Anaest Scand 1995; 39, Suppl 107: 123-28.
- Singer RB, Hastings AB. An improved clinical method for the estimation of disturbances of the acid-base balance of human blood. Medicine (Baltimore) 1948; 27: 223-42.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









