Printed from acutecaretesting.org
June 1999
Application of hemoglobin derivatives in STAT analysis
INTRODUCTION
The classic “blood gas” (pH, pCO2, pO2) has been and still is the best laboratory-based means for assessing acid-base status and gas exchange in the urgent care setting. However, recent technological advances allow reliable measurements of blood gases, total hemoglobin, electrolytes, oxygen saturation, and related quantities such as carboxyhemoglobin and methemoglobin, to be made on the same sample and on the same analyzer.
These in turn allow accurate determination of, for example, the oxygen content. These analytical realities force a shift in the clinical assessment paradigm, especially for patients with unknown, uncertain, or ambiguous clinical history.
One of the most important components of critical care assessment is oxygenation, the most common laboratory means of assessment being the measurement of pO2(aB). The challenge of this diagnostic approach lies in the complexity of oxygenation which is classically thought of as having four general aspects: ventilation/pulmonary perfusion, systemic and regional blood flow (cardiac output vs splanchnic flow), transport capacity, and release/utilization of oxygen in the metabolizing tissue.
These, while conceptually separate, are functionally inseparable. They are also highly significant diagnostically, especially on a “rule in/rule out” basis in critical care situations. Measurement of some of the key quantities associated with oxygenation has not been widely available until recently.
Consequently, a plethora of alternate clinical and laboratory approaches for assessing oxygenation status has become widespread, possibly to the detriment of more exact clinical science. One might assume, then, that many practicing physicians and clinical biochemists are not fully aware of the significance, usefulness, and limitations of measurements now commonly available.
OXYGENATION QUANTITIES
For the laboratorian, hypoxemia is commonly defined based on a patient’s arterial blood oxygen tension, pO2(aB), or oxygen content, ctO2(aB), while the clinical condition of interest, hypoxia, represents the qualitative extent of oxygenation in the metabolizing tissue.
Laboratory estimation of hypoxia is made on the basis of measurements on blood, combined with an understanding of the physiology and pathology of the patient’s situation. Thus an oxygen saturation obtained in the Emergency Department may be 80 % (0.8) or 99 % (0.99), and indicative of widely disparate conditions in the acute overdose patient, the trauma victim, or the renal stone patient.
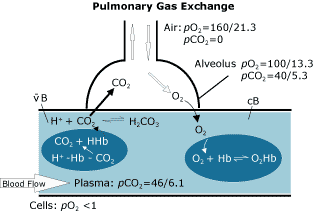
FIG. 1
Though its characteristics are commonly understood, the partial pressure or tension of oxygen in arterial blood, pO2(aB), is included here for completeness (see Fig. 1). The tension of oxygen is the driving force in moving oxygen from one compartment of the body to another (e.g. from the alveoli of the lungs to the pulmonary capillaries), rather than being an indicator of the amount of oxygen being moved.
While a typical level for pO2(aB) in young, healthy adults is > 12.7 kPa (95 mmHg), therapeutic intervention may not be taken until pO2 is somewhat lower, depending on the complete clinical picture of oxygen delivery. The oxygen tension of arterial blood, pO2(aB), is reduced with impaired lung function (e.g. chronic obstructive pulmonary disease or pneumonia) when there is a decrease in the inspired oxygen tension (e.g. at high altitudes or in the presence of other gases), or with age.
Measures to improve pO2(aB) include optimizing the mechanical ventilation and otherwise increasing the inspired oxygen tension, FO2(I). Along with the total hemoglobin concentration, pO2 of arterial blood may be the primary indication of oxygenation status for most patients, though many clinicians use pulse oximetry saturation measurements as a practical assessment of oxygenation, despite its limitations.
In many cases oxygenation assessment can be accomplished using arterial blood gases and clinical observation alone, although the more critically ill require more exhaustive assessment. Interestingly, with the widespread availability of pulse oximeter saturation measurements, there is a tendency to rely on, these measurements in a wider range of urgent clinical situations, than may actually be warranted.
The total hemoglobin concentration (ctHb) is a measure of the total potential oxygen-carrying capacity, i.e. the sum of all the hemoglobin fractions. Hemoglobin is typically quantified as a part of a hematology profile, by converting all forms of hemoglobin - O2Hb, HHb, plus the non oxygen-carrying or dysfunctional hemoglobins (dysHb) - to a single stable compound.
The total absorbance of this chromophore, usually a methemoglobin derivative, is measured and compared to a reference method (e.g. NCCLS Document H15-A) or material whose values are traceable to that method.
As well as total hemoglobin, the hemoglobin fractions that are of clinical interest in oxygenation assessment are easily measured by multiwavelength photometry, often referred to as oximetry (CO-oximetry, hemoximetry). The concentration of each Hb derivative present is directly measured and added, to provide ctHb.
ctHb = cO2Hb + cHHb + cdysHb
By tradition, the derivatives are not reported as concentrations but as ratios (fractions) of ctHb.
The oxygen-carrying capacity of hemoglobin is given by the sum of oxyhemoglobin and deoxyhemoglobin. If no significant amounts of the dyshemoglobins are present, the total hemoglobin is a reliable indicator of the oxygen-carrying capacity of hemoglobin.
Two hemoglobin fractions need to be specified to allow their contrast with clinically more useful terms:
1. Fractional oxyhemoglobin, FO2Hb, is defined as the fraction of oxygenated hemoglobin in relation to the total of all hemoglobins present (including the non-oxygen binding dyshemoglobins)
FO2Hb = cO2Hb =
ctHb
cO2Hb
cO2Hb + cHHb + cCOHb + cMetHb
2. Fractional deoxyhemoglobin, FHHb, is defined as the fraction of unoxygenated hemoglobin to total hemoglobin:
FHHb = cHHb =
ctHb
cHHb
cO2Hb + cHHb + cCOHb + cMetHb
In contrast to FO2Hb, the oxygen saturation, sO2, is the fraction of oxygenated hemoglobin in relation to the amount of hemoglobin capable of carrying oxygen:
sO2 = cO2Hb
cO 2Hb + cHHb
With the arterial oxygen tension, the oxygen saturation of arterial blood, sO2(aB), is a corollary indicator of the adequacy of the lungs to get oxygen to the blood.
FO2Hb is often erroneously referred to as “saturation”, despite the fact that FO2Hb and sO2 are different quantities as is obvious from the equations. Because most patients probably do not have significant amounts of the dyshemoglobins, the numerical values of the two quantities may be very similar, a fact that reinforces the misuse.
Oxygen saturation, sO2, may be determined by several methods. It is, therefore, important for laboratorians and clinicians to be aware that the reliability and accuracy depend on the analytical method used; each has its own analytical limitations.
One example is the potentially severe limitations of sO2 reported from a stand-alone blood gas analyzer, estimated from a measured pO2 and deduced from a standard oxyhemoglobin dissociation curve (ODC).
Another example is the analytical limitations of oxygen saturation measured by pulse oximetry, sO2(p), compared to oxygen saturation determined on a multiwavelength photometer on an arterial blood sample, sO2(aB).
Non Oxygen-Binding Hb Derivatives
Dyshemoglobins are those hemoglobin derivatives incapable of reversibly combining with molecular oxygen under physiological conditions. The most common dyshemoglobins of clinical significance, carboxyhemoglobin (COHb) and methemoglobin (MetHb), are usually measured and reported separately.
Only very rarely does sulfhemoglobin (SulfHb) become quantitatively significant. Other unnamed, nonspecific dyshemoglobins (dysxHb) may be present, but these nearly always represent less than a fraction of 1 % of the total.
Although frequently the major dyshemoglobins (COHb and MetHb) are only thought of as being significant in circumstances such as suicide attempts or a genetic lack of methemoglobin reductase, unexpected and clinically significant elevations can occur in smokers, AIDS therapy, and through domestic exposure to certain toxic agents, among others. The selected bibliography is useful in pursuing this subject in detail. [3,4,6-16,19,20]
The reference range for FCOHb in non-urban, non-smoking subjects is typically cited as < 0.01 (< 1.0 %), and for FMetHb, about < 0.015 (< 1.5 %).
There is no acute clinical impact of slight elevations above these ranges, and moreover, multiwavelength photometers (hemoximeters, CO-oximeters) are probably unreliable devices for measuring or differentiating between levels of FCOHb and FMetHb below 0.02 to 0.025 (2 - 2.5 %) in individual patients. FCOHb in smokers can normally rise to as high as 0.06 to 0.08 (6 - 8 %), whereas that of urban, non-smokers lies somewhere in between.
The initial assessment of patients with hypoxic symptoms typically includes measurement of the blood gases, and should also include measurement of the hemoglobin derivatives. After the initial measurement, no further follow-up on the Hb derivatives is routinely required. If COHb or MetHb are significantly elevated, immediate action may be taken to lower the fractions of those quantities, or to increase the amounts of oxygen dissolved in blood, as deemed appropriate.
Oxygen Content or Concentration of Total Oxygen
The concentration of total oxygen, more commonly referred to as oxygen content, is the sum of oxygen bound to hemoglobin and that physically dissolved in blood:
ctO2 = Hb bound O2 + Dissolved O2 = ctHb ·FO2Hb · H1+ pO2 · α
1H represents Hueffner's coefficient for the total oxygen binding capacity of the hemoglobin molecule, i.e. the amount of oxygen bound per unit O2Hb. In SI units, the value is 1 mmol O2/mmol O2Hb. In conventional units this corresponds to 1.39 mL oxygen (at standard temperature and pressure) per g O2Hb, a value which is confirmed by X-ray crystallography of the hemoglobin molecule.
However, many clinical tests still refer to its value as being 1.34 (mL O2Hb). α is the plasma solubility coefficient/units conversion of oxygen.
The concentration of total oxygen measured on an arterial blood sample, ctO2(a), is the primary indicator of the blood’s oxygen delivery capability. On a practical basis, a hemodynamically stable patient with known baseline values for ctO2 and the dyshemoglobins can be managed with knowledge of the basic blood gases and ctHb.
For most situations, the dissolved oxygen is analytically and clinically unimportant. Nevertheless at very low levels of hemoglobin, in patients receiving hyperbaric oxygen therapy or in significantly hypothermic patients, dissolved oxygen may be a very significant contributor to oxygen transport.
The oxygen content of blood was originally measured directly by Van Slyke, using manometric techniques. Subsequently, spectrophotometric means of measuring hemoglobin derivatives were developed, based on the fact that slight differences in the chemical structure of different hemoglobin derivatives result in quantitatively different light-absorbing characteristics.
As noted earlier, current technology enables the measurement of oxygen-bound hemoglobin, deoxyhemoglobin, and individual dyshemoglobins. The sum of all these quantities is the total hemoglobin, which allows for the determination of the fractions of the various Hb derivatives, while the combination of oxyhemoglobin and deoxyhemoglobin enable direct derivation of sO2.
When combined with the measured oxygen tension and the solubility constant for oxygen, for all clinical purposes, the quantity given by the equation for the oxygen content in the section above is equivalent to the original manometric determination. Measures to improve ctO2 focus on improving one or more of its determinants: ctHb, FO2Hb, and pO2.
The intrapulmonary shunt fraction (Qsp/Qt or FShunt) represents the fraction of mixed venous blood that is not exposed to fully-functioning alveoli during passage to the left side of the heart.
The model is based on the simplifying assumption that one part, Qsp, of the total blood flow, Qt, is shunted around the lungs receving no oxygen, whereas the remaining part of the blood is fully equilibrated with alveolar air. Using ‘cB’ for pulmonary capillary blood and ‘vB’ for mixed venous blood, simple mathematics can then provide the equation:
(Qsp/Qt) = [ctO2(cB) - ctO2(aB) ]
[ctO2(cB) - ctO2(vB)]
The calculation is based on measured values of ctO2(aB), ctO2(vB), and the barometric pressure. Additionally FO2(I) and the patient temperature are required to estimate ctO2(cB) from the alveolar equation.
An estimated intrapulmonary shunt fraction, Qsp/Qt(est) or Fshunte, is frequently determined by the analyzer or the clinician when a mixed venous sample of blood cannot be obtained. In that situation a specific value for the difference between arterial and mixed venous content is assumed. The Qsp/Qt(est) is highly correlated with the true shunt determination, with correlation values > 0.9, but it is only reliable if the patient is hemodynamically stable.
The shunt fraction can be elevated in both chronic and acute disease, and a sudden increase in the value reported can produce serious consequences. Analytically and clinically, in the assessment of the shunt fraction its accuracy is dependent on the accuracy of the oxygen content values used. A shunt effect is demonstrated when arterial pO2 tensions are less than ideal, but is generally not significant if levels are still high enough (> 10.7 kPa) to effectively saturate the blood hemoglobin.
The shunt fraction determined under the condition of an increased inspired oxygen fraction, FO2(I), is an important means of differentiating between pulmonary or cardiac insufficiency as the cause of hypoxemia.
MEASUREMENT OF Hb AND ITS DERIVATIVE QUANTITIES
Optical technology provides us with the same information as the original manometric technology, based on the distinct difference in the light absorption spectrum of oxygenated hemoglobin.
Measurement of the light absorbed by a specimen at two selected wavelengths of emitted light, provides unique solutions analogous to the ratio of light absorbed by each component, to directly obtain the saturation. This works well for specimens which have no dyshemoglobins, and underlies the principle of pulse/cutaneous oximetry.
Oximetry: Hemoglobin Derivatives and tHb
A common occurrence in clinical practice is that in addition to oxyhemoglobin and deoxyhemoglobin, the blood collected contains some level of other hemoglobin derivatives, particularly COHb and MetHb. Fig. 2 represents the absorption spectra of equimolar amounts of possible hemoglobin derivatives.
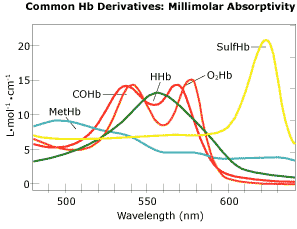
FIG. 2
To obtain the most reliable value for oxygen saturation, determine the oxygen transport quantities already discussed, or assess the cause of any clinical hypoxia, simple oximetry alone is inadequate. However, using the same optical principles, all the significant hemoglobin derivatives may be measured in a suitable photometer capable of measuring the absorbance at four or more wavelengths (one wavelength for each of the four typical Hb derivatives).
Since this approach takes all the likely hemoglobin derivatives into account, the photometer will be capable of measuring, by addition, the total hemoglobin and thus the fraction of each derivative. In addition, other derived values can be reliably determined based on independent knowledge of the molecular structure of hemoglobin, and on these measurements.
Multicomponent Hemoglobin Analysis
An algebraic axiom states that in order for one to obtain a unique set of solutions for a set of “n” unknowns, one must have “n” independent equations. This is fundamental to the simultaneous multicomponent analysis used in blood oximetry.
Since all blood specimens will have at least two hemoglobin derivatives (oxyhemoglobin and deoxyhemoglobin), and many will have small amounts of two others (carboxyhemoglobin and methemoglobin), a basic hemoglobin photometer needs to measure at four wavelengths of light. The corresponding set of equations is referred to by mathematicians as a determined matrix.
Some manufacturers have developed oximeters that incorporate additional wavelengths of light to account for absorbance due to turbidity, the presence of sulfhemoglobin, the interfering effects of hemoglobin F, and to assess inherent measurement errors such as those caused by extraneous light absorbing dyes, thermal effects, or other factors. Systems that incorporate specific additional wavelengths for error detection are said to be “overdetermined”.
The specific advantage with overdetermined systems is not, typically, any significant difference in the clinical accuracy on the specimens measured. Rather, when unexpected conditions exist such as lipemia, the presence of unexpected therapeutic dyes, or even wavelength fluctuations resulting from thermal changes in the system, these effects will be either accounted for in the final result reported, or an error will be signaled to the operator of the overdetermined system. The determined system however, will simply report the compromised result.
Technical details may be found in [2].
CLINICAL APPLICATIONS OF HEMOGLOBIN DERIVATIVES
As a direct effect of the presence of a dyshemoglobin, the oxygen-carrying capacity of the hemoglobin molecule is reduced. Thus the total hemoglobin or hematocrit cannot be used as an index of the ability to transport adequate oxygen. In addition, for both COHb and MetHb, the oxyhemoglobin dissociation curve is shifted to the left due to structural changes in the Hb tetramer.
This indicates that at any given saturation level of the available hemoglobin, the oxygen tension is lower and hemoglobin will tend to hold on to the O2 that is bound. It is worthwhile considering that in cases of equivalent anemia, an increase in 2,3-DPG is often seen. This shifts the ODC to the right and allows more oxygen to be released at the same hemoglobin oxygen saturation.
This dual effect of lowering the available oxygen in the presence of dyshemoglobins can clearly exacerbate issues related to reduced oxygen availability or increased oxygen demand. This makes accurate evaluation of even limited elevations of dyshemoglobins of interest. The key to any clinical interpretation is measurement of the significant dyshemoglobins, COHb and MetHb, in any initial evaluation of apparent hypoxia, unless the patient’s history can unequivocally rule out the dyshemoglobins.
Methemoglobin Toxicology: Cellular Hypoxia
Methemoglobin is hemoglobin in which the ferrous iron has been converted to ferric iron. The ferric iron cannot reversibly combine with oxygen, and thus MetHb does not transport oxygen. In normal subjects, the methemoglobin’s ferric iron which is formed naturally is subsequently reduced to the ferrous state by enzymes known as methemoglobin reductases.
This process keeps MetHb at very low levels. In fact, the typical MetHb levels seen are below the true detection limit of multiwavelength hemoglobin photometers (oximeters), leading some laboratories to list normal values as “less than x %” and to report them in the same manner.
Genetic reductase deficiency or exposure to oxidizers (or both combined) can lead to elevated levels of MetHb. One should refer to the selected bibliography [Ref] for an indication of the numerous clinical situations in which MetHb may be a significant contributor to hypoxia. Many of these situations are unexpected, and possibly inadequately recognized and treated.
It should also be noted that some situations involving chronic or acute exposure to oxidizers do not always have simple clinical solutions. In some cases oxidizers may diffuse into tissues and inhibit various metabolic pathways, including oxidative phosphorylation, subsequently directly affecting oxygen utilization.
Selected Sources of MetHb Elevations
- Local anesthetics: the Caines
- Industrial exposure: nitrous fumes, burns, nitrogen salts
- Plastics and other building materials
- Cyano-compounds
- Nitrates and nitrites as used in the explosives industry and in agriculture.
- Acetylene torches as used in repair and manufacture.
- Silage production on farms
- NO therapy for pulmonary hypertension
Clinical CO Toxicology
In analogy with oxygen, carbon monoxide is capable of reversible, covalent binding to hemoglobin. As binding takes place at the same sites, O2 and CO compete for the reversible association to hemoglobin. The affinity of CO to Hb is more than 200 times greater than that of O2 to Hb, thus minute concentrations of CO gas in the atmosphere can displace a significant portion of the oxygen from hemoglobin.
In addition to simple displacement, CO can enter the cell and inhibit the oxidative pathway. Alone or in combination, these effects can bring about tissue hypoxia, acidosis, and depression of the central nervous system, affecting respiration, carbon dioxide retention, and acid formation - a degenerative cycle.
Clinical Issues in CO Toxicity
- High pCO leading to O2 displacement
- High metabolism (fever, exertion) leading to increased O2 demand
- Anemia, other dysHb lea-ding to lower BO2
- Other clinical conditions (cardiac insufficiency, trauma, burns, pulmonary irritation)
- Post exposure interval before treatment vs FCOHb level
- Treatment requirements (e.g. high O2 vs. hyperbaric O2)
- In vivo CO production (dichloromethane, methylene chloride)
- Effect synergy (demand increased; BO2(aB) decreased)
As an example of the need for complete assessment of any clinical situation, consider the exposure of a subject to automobile exhaust. Legally permissible CO levels of exhaust gas range from < 0.5 % to > 4.0 %, both limits ultimately capable of complete saturation of hemoglobin. However, while not significantly displacing oxygen in the atmosphere, carbon monoxide rapidly binds to Hb due to its much higher affinity.
In addition to these aspects of the toxicological process, the clinical symptoms and signs may be delayed or covered up by more pressing clinical realities. This is frequently the case with fire victims, where burns, lung irritation, and other traumatic injuries may be the most apparent issues, and the definitive therapy obvious.
However, when the CO-related issues become more apparent, the CO levels may well be back in the normal range, leaving the exact cause unknown and the treatment less than adequate.
There is an increasing awareness that the most appropriate course of action when CO is involved, is to measure both COHb and MetHb, if only to rule out the synergistic effects.
Additionally, if COHb is elevated, whether or not MetHb is also elevated and especially if there is any history of unconsciousness, hyperbaric oxygen therapy may need to be considered. As may be seen from Fig. 3, the elimination of CO under hyperbaric conditions is extremely effective in reducing COHb levels compared to room air and 100 % oxygen.
HEMOGLOBIN DERIVATIVES IN CRITICAL CARE - A SUMMATION
In assessing the use of CO-oximetry in the critical care environment, several factors need to be taken into account. As we have seen, it is essential that measured values for oxygen content are reported for clinical situations involving the evaluation of sepsis and multiple organ failure (MOF).
Sepsis and MOF are some of the major causes of morbidity in intensive care and they can develop rapidly, with mortality rates approaching 50 %. As supplier of the information important for clinical assessment, the laboratory must provide the right quantity of data measured correctly. We have seen that calculations of saturation from a stand-alone BGA are totally insufficient, as are measured saturations when dyshemoglobins are present, or if 2,3-DGP is abnormal.
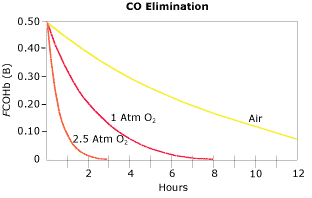
FIG. 3
In emergency medicine, similar issues hold true. Saturation and derived oxygen content obtained by pulse oximetry give little or no indication of the presence of dyshemoglobins. Coupled with the various potential causes for elevated dyshemoglobins, some of which are frequently unrecognized, measurement of hemoglobin derivatives is important, if only to rule out dyshemoglobinemias as contributors to the clinical picture, and to check the displacement of the ODC.
The last point is important to remember - the contributory effect. No single dyshemoglobin may be the cause of all clinical observations, but each elevation when coupled with other factors affecting oxygen transport and delivery, can result in increased morbidity/mortality. Measurement is the key.
With the advent of more intensive modes of therapy, and with new initiatives for understanding and controlling the real cost of care, it seems prudent to include multiwavelength photometry (CO-oximetry) as a routine part of initial laboratory studies for emergency room care, which in the past have warranted a “blood gas” evaluation. Multiwavelength photometry would also be useful for following patients under intensive therapy, where the risk of sepsis and MOF is high.
These, then, are the major quantities that may be determined by the laboratory which are used by the clinician to assess the oxygenation status. Each measurement tells a different part of the clinical story. Standardization of the terminology and symbols used in reporting, and the calculations for estimating the various quantities and the analysis itself, are the major responsibilities of the laboratory scientist.
The integration of clinical requirements with analytical capabilities is necessary to assure proper patient care.
References+ View more
- Brunelle JA, Degtiarov AM, Moran RF, Race LA. Simultaneous measurement of total hemoglobin and its derivatives in blood using CO-oximeters: Analytical principles; their application in selecting analytical wavelengths and reference methods; a comparison of the results of the choices made. Scand J Clin Lab Invest 1996; 56, Suppl 224: 47-69.
- Brunnelle JA, Degtiarov AM, Moran RF, Race LA. CO-oximetric measurement of oxyhemoglobin, deoxyhemoglobin, and dyshemoglobins in blood: Effects of analytical wavelength and reference method selection. Lab Hematol 1995; 1, 2: 161-64.
- Chakraborty TK. Filshie JRA, Lee MR. Methaemoglobinaemia produced by phenazopyridine (pyridium) in a man with chronic obstructive airway disease. Scottish Med J 1987; 32: 185-86.
- Collins J. Methemoglobinemia as a complication of 20 % benzocaine spray for endoscopy. Gastroenterology 1990; 98, 1: 211-13.
- Degen BR, Moran RF. Comparison and assessment of blood gas related quantities including base excess, the gas exchange indices and temperature corrected pH/pO2/pCO2 , as defined in approved NCCLS standard C12-A, using a computer simulation of input variables. Scand J Clin Lab Invest 1996; 56, Suppl 224: 89-106.
- DiMarco AT. Carbon monoxide poisoning presenting as polycythemia. N Engl J Med 1988: 874.
- Erstad BL. Dapsone-induced methemoglobinemia and hemolytic anemia. Clin Pharm 1992; 11: 800-05.
- Ferraro L, Zeichner S, Greenblott G, Groeger JS. Cetacaine-induced acute methemoglobinemia. Anes 1988; 69,4: 614-15.
- Hart IK, Kennedy PGE, Adams JH, Cunningham NE. Neurological manifestation of carbon monoxide poisoning. Postgrad Med J 1988; 64: 213-16.
- Hebbel RP, Eaton JW, Modler SV, Jacob HS. Extreme but asymptomatic carboxyhemoglobinemia and chronic lung disease. JAMA 1978; 239, 24: 2584-86.
- Hoffman RS, Sauter D. Methemoglobinemia resulting from smoke inhalation. Vet Hum Toxicol 1989; 31, 2:168-70.
- Huff JS, Kardon E. Carbon monoxide toxicity in a man working outdoors with a gasoline-powered hydraulic machine. N Engl J Med 1989: 1564.
- Kales AI. Carbon monoxide intoxication. Am Family Physician1993; 48, 6: 1100-04.
- Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med 1987; 146,1: 52-56.
- Kross BC, Ayebo AD, Fuortes LJ. Methemoglobinemia: nitrate toxicity in rural America, Am Family Physician 1992; 46,1: 183-88.
- Laney RF, Hoffman RS. Methemoglobinemia secondary to automobile exhaust fumes. Am J Emerg Med 1992; 10,5: 426-28.
- Mahoney JJ, Vreman HJ, Stevenson DK, Van Kessel. Measurement of carboxyhemoglobin and total hemoglobin by five specialized spectrophotometers (CO-oximeters) in comparison with reference methods. Clin Chem 1993; 39, 8: 1693-1700.
- Severinghaus JW, Xu F, Spellman MJ. Benzocaine and methemoglobin: recommended actions. Anesthes 1991; 74,2: 385-86.
- Sokal JA, Kralkowska E. The relationship between exposure duration, carboxyhemoglobin, blood glucose, pyruvate and lactate and the severity of intoxication in 39 cases of acute carbon monoxide poisoning in man. Arch Toxicology 1985; 57, 196-99.
- Thom SR, Keim LW. Carbon monoxide poisoning: a review epidemiology, pathophysiology, clinical findings, and treatment options including hyperbaric oxygen therapy. Clin Tox 1989; 27,3: 141-56.
- Vreman HJ, Stevenson DK. Carboxyhemoglobin determined in neonatal blood with a CO-oximeter unaffected by fetal oxyhemoglobin. Clin Chem 1994; 40,8: 1522-27.
- Yukawa N, Suzuoka T, Saito T, et al. Data processing in CO-oximeters that use overdetermined systems., Clin Chem 1997; 43,1: 189-91.
- Zijlstra WJ, Maas AHJ, Moran RF. Definition, significance and measurement of quantities pertaining to the oxygen carrying properties of human blood. Scand J Clin Lab Invest 1996; 56, Suppl 224: 27-45.
References
- Brunelle JA, Degtiarov AM, Moran RF, Race LA. Simultaneous measurement of total hemoglobin and its derivatives in blood using CO-oximeters: Analytical principles; their application in selecting analytical wavelengths and reference methods; a comparison of the results of the choices made. Scand J Clin Lab Invest 1996; 56, Suppl 224: 47-69.
- Brunnelle JA, Degtiarov AM, Moran RF, Race LA. CO-oximetric measurement of oxyhemoglobin, deoxyhemoglobin, and dyshemoglobins in blood: Effects of analytical wavelength and reference method selection. Lab Hematol 1995; 1, 2: 161-64.
- Chakraborty TK. Filshie JRA, Lee MR. Methaemoglobinaemia produced by phenazopyridine (pyridium) in a man with chronic obstructive airway disease. Scottish Med J 1987; 32: 185-86.
- Collins J. Methemoglobinemia as a complication of 20 % benzocaine spray for endoscopy. Gastroenterology 1990; 98, 1: 211-13.
- Degen BR, Moran RF. Comparison and assessment of blood gas related quantities including base excess, the gas exchange indices and temperature corrected pH/pO2/pCO2 , as defined in approved NCCLS standard C12-A, using a computer simulation of input variables. Scand J Clin Lab Invest 1996; 56, Suppl 224: 89-106.
- DiMarco AT. Carbon monoxide poisoning presenting as polycythemia. N Engl J Med 1988: 874.
- Erstad BL. Dapsone-induced methemoglobinemia and hemolytic anemia. Clin Pharm 1992; 11: 800-05.
- Ferraro L, Zeichner S, Greenblott G, Groeger JS. Cetacaine-induced acute methemoglobinemia. Anes 1988; 69,4: 614-15.
- Hart IK, Kennedy PGE, Adams JH, Cunningham NE. Neurological manifestation of carbon monoxide poisoning. Postgrad Med J 1988; 64: 213-16.
- Hebbel RP, Eaton JW, Modler SV, Jacob HS. Extreme but asymptomatic carboxyhemoglobinemia and chronic lung disease. JAMA 1978; 239, 24: 2584-86.
- Hoffman RS, Sauter D. Methemoglobinemia resulting from smoke inhalation. Vet Hum Toxicol 1989; 31, 2:168-70.
- Huff JS, Kardon E. Carbon monoxide toxicity in a man working outdoors with a gasoline-powered hydraulic machine. N Engl J Med 1989: 1564.
- Kales AI. Carbon monoxide intoxication. Am Family Physician1993; 48, 6: 1100-04.
- Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med 1987; 146,1: 52-56.
- Kross BC, Ayebo AD, Fuortes LJ. Methemoglobinemia: nitrate toxicity in rural America, Am Family Physician 1992; 46,1: 183-88.
- Laney RF, Hoffman RS. Methemoglobinemia secondary to automobile exhaust fumes. Am J Emerg Med 1992; 10,5: 426-28.
- Mahoney JJ, Vreman HJ, Stevenson DK, Van Kessel. Measurement of carboxyhemoglobin and total hemoglobin by five specialized spectrophotometers (CO-oximeters) in comparison with reference methods. Clin Chem 1993; 39, 8: 1693-1700.
- Severinghaus JW, Xu F, Spellman MJ. Benzocaine and methemoglobin: recommended actions. Anesthes 1991; 74,2: 385-86.
- Sokal JA, Kralkowska E. The relationship between exposure duration, carboxyhemoglobin, blood glucose, pyruvate and lactate and the severity of intoxication in 39 cases of acute carbon monoxide poisoning in man. Arch Toxicology 1985; 57, 196-99.
- Thom SR, Keim LW. Carbon monoxide poisoning: a review epidemiology, pathophysiology, clinical findings, and treatment options including hyperbaric oxygen therapy. Clin Tox 1989; 27,3: 141-56.
- Vreman HJ, Stevenson DK. Carboxyhemoglobin determined in neonatal blood with a CO-oximeter unaffected by fetal oxyhemoglobin. Clin Chem 1994; 40,8: 1522-27.
- Yukawa N, Suzuoka T, Saito T, et al. Data processing in CO-oximeters that use overdetermined systems., Clin Chem 1997; 43,1: 189-91.
- Zijlstra WJ, Maas AHJ, Moran RF. Definition, significance and measurement of quantities pertaining to the oxygen carrying properties of human blood. Scand J Clin Lab Invest 1996; 56, Suppl 224: 27-45.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









