Printed from acutecaretesting.org
January 2011
Critical values in laboratory medicine
THE CONCEPT OF CRITICAL VALUES
It was Lundberg and colleagues working at the clinical laboratories of the Los Angeles County/University of Southern California Medical Center who pioneered a systematic approach to the identification and communication of dangerously abnormal test results.In 1972 they coined the term "critical (panic) value", which they defined as "one that represents a pathophysiological state at such variance with normal as to be life-threatening unless something is done promptly, and for which some corrective action could be taken" [1,2].
Having established a list of critical (panic) values that accorded, to their satisfaction, with the definition, the system placed responsibility on medical technologists to identify and verify critical (panic) value test results and then communicate them urgently to a "responsible individual".
The "Lundberg" concept has since been adopted in one form or another by laboratories across the world. The term "critical (panic) value" has largely been abandoned in favor of "critical value" [17].
The term "critical limits" refers to the analyte-specific set limits that define a test result as a "critical value".
Most analytes appearing on a critical-test list have both high and low critical limits but for some (e.g. neonatal serum bilirubin) only a high value could be considered critical and for others (e.g. pO2(a)) only a low value is considered critical.
A distinction must be made between a critical-value test result as defined by Lundberg, and an urgent (or STAT) test result that in some sense is of course also "critical".
The decision to nominate a particular test request urgent is made by the attending doctor based on consideration of the patient's possible (or certain) critical condition.
Such test requests are processed urgently in the laboratory and the result communicated urgently, irrespective of whether the result is normal or abnormal. A critical-value test result, by contrast is identified in the laboratory after non-urgent (or urgent) analysis, without knowledge of the patient's condition.
By definition, a critical-value result is grossly abnormal and the decision to communicate the result urgently is made not by the attending doctor who requested the test, but by laboratory staff, based on preset critical limits.
The process of communicating critical-value test results and communicating urgent (STAT) test results may be the same.
This article is concerned only with critical-value test results, although it is worth noting that there might be overlap between policy relating to critical-value reporting and policy relating to urgent (STAT) test reporting.
The concept of critical values was first endorsed by US regulatory authorities in the Clinical Laboratory Improvement Amendments (CLIA) of 1988 which stated that "laboratories must develop and follow written procedures for reporting life-threatening laboratory results or panic values" [3].
More recently the Joint Commission on Accreditation of Health Care Organizations (JCAHO), responsible for laboratory accreditation in the US, identified effective reporting of laboratory critical values as a National Patient Safety Goal (NSPG.02.03.01) [4].
Documentation of laboratory policy relating to critical-value reporting is mandated by JCHO [4]. The internationally agreed standards for medical laboratory competence and quality, ISO 15189:2007, which has been adopted by national laboratory accreditation agencies in Europe and beyond, includes the requirement that critical values are notified urgently.
Although regulatory authorities acknowledge that timely reporting of critical values is essential to the overall quality of the work of clinical laboratories, and laboratory accreditation in many parts of the world now demands a regularly reviewed locally written policy regarding critical values, there is apart from a single paper written under the auspices of the American College of Clinical Pathologists (ASCP) in 1997 [5], and "consensus recommendations" from the Italian Society of Laboratory Medicine [6] (available only in Italian), very little formal guidance on the detail of such policy.
So, for example, there is no internationally or nationally agreed list of laboratory tests that warrant assignment of critical limits, and even for those laboratory tests that most agree warrant critical limits there is lack of consensus on the critical limits that should be applied.
The formulation and review of all aspects of critical value policy is thus a matter for local laboratory/healthcare facility management.
FORMULATING/REVIEWING A LOCAL CRITICAL-VALUE POLICY
Aspects that need to be addressed in discussing, formulating or reviewing policy for critical values include:- the list of tests that warrant the term "critical test" (i.e. those tests for which an abnormal result could indicate a life-threatening state if immediate corrective action is not taken)
- the critical limits to be applied to each critical test (i.e. the value which, if exceeded, is deemed to constitute a potentially life-threatening state)
- the laboratory process that ensures identification of critical values
- the laboratory personnel who are to be responsible for communicating critical values
- the mode of communication (e.g. telephone, pager, computer)
- the staff who are to be responsible for receipt of critical values
- procedure directed at ensuring the critical-value message has been accurately received. (The Joint Commission require that critical-value results be read-back [4]).
- appropriate/acceptable time frame for the period between identification of critical value in the laboratory and receipt of that critical value by the member of clinical staff currently responsible for patient care
- procedures to be adopted if laboratory staff are unable to contact an appropriate member of staff
- documenting the communication process and thereby monitoring the effectiveness of the policy
A successful critical-value policy depends on the cooperative effort of a range of healthcare workers, including laboratory staff, nursing and medical staff both in the hospital and the community, and sometimes ward clerks and medical secretaries.
It is important that representatives of all stakeholders are involved in the formulation and review of the policy, and that all staff involved in implementing the policy are well educated in their role and embrace the policy [7].
For example, the list of critical tests should not be imposed but reflect the expressed needs of the clinical community that use the laboratory, and critical limits should reflect current local expert opinion within the laboratory and medical community about what constitutes an immediate life-threatening state as envisaged by Lundberg.
The aim of a critical-value policy is to ensure that no patient suffers as a result of delay in appropriate treatment for a potentially life-threatening condition that has been identified by laboratory testing.
But equally, a successful critical-value policy ensures that valuable resources (laboratory, nursing and medical staff time) are not wasted in urgently communicating laboratory test results that, despite perhaps gross abnormality, do not indicate the need for immediate corrective action.
A potential problem of information overload for busy doctors is inherent in the concept of critical values; Lundberg acknowledged this [2]. A number of critiques examining the concept of critical values and associated practical problems to be addressed in formulating policy are available in the literature [2,7-10].
Additionally, published surveys and audits of critical-value policy [11-19] provide some detail of how the concept of critical values is applied and give an indication of best, or at least most common practice.
THE CRITICAL-TEST LIST
Most surveys of critical-value policy [11-20] have been conducted in the US. There have also been at least three European studies, one in the UK [13] relating to clinical chemistry tests only, and two in Italy [19,20].
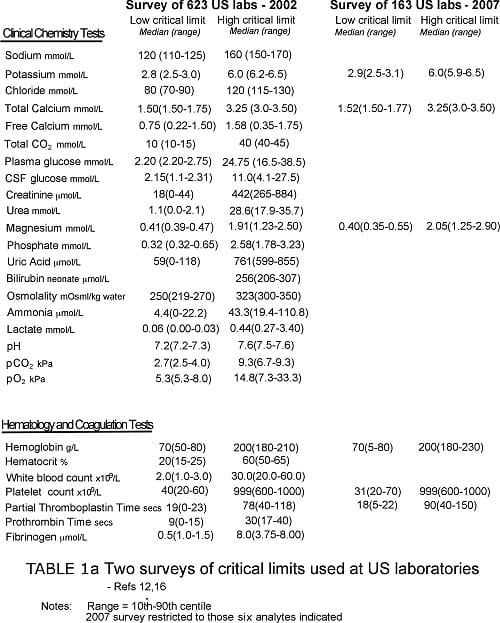
The largest and most comprehensive of these studies, published in 2002, surveyed the detail of critical-value policy at 623 laboratories across the US [12]. It revealed marked variability in what constitutes a critical test.
Almost all laboratories included the following analytes on their critical-test list: sodium, potassium, glucose, calcium, hemoglobin/hematocrit, white-cell count and platelet count.
In addition, positive blood and CSF culture results were deemed a critical value in nearly all laboratories. A majority of laboratories included a further seven chemistry tests/analytes (blood gases, urea, creatinine, magnesium, phosphorous, neonatal bilirubin, tests of therapeutic drug monitoring and two further hematology tests (activated partial thromboplastin time [APPT] and prothrombin time [PT]).
Other analytes appearing on a minority of critical-test lists included ammonia, osmolality, lactate, chloride, fibrinogen and amylase but there were many others. In fact a further 65 tests were included on the critical-test list of at least one laboratory.
Some of these, including urine crystals, mean cell volume (MCV) and haptoglobin, are clearly not critical tests as conceived by Lundberg, and the presence on a critical-test list of many other tests amongst these 65 would be difficult to justify.
A survey of 92 UK clinical chemistry laboratories [13] revealed equal lack of consensus with regard to critical-test lists, with 27 laboratories listing fewer than 10 critical tests and others listing in excess of 20.
CRITICAL LIMITS
National surveys reveal equal lack of consensus when critical limits are examined. For example, Howanitz et al [12] found that the mean low critical limit for plasma sodium among 623 US laboratories was 120 mmol/L, but this ranged from 110 mmol/L to 125 mmol/L, and that for potassium ranged from 2.5 mmol/L to 3.0 mmol/L.
The upper critical limit for serum bilirubin among neonates ranged from 206 mmol/L to 307 mmol/L.
The UK survey [13] found even greater disparity. Low critical limit for sodium ranged from 110 to 130 mmol/L and high limit from 147 to 170 mmol/L. Low critical limit for glucose ranged from 1.5 to 3.3 mmol/L and upper limit ranged from 15.0 to 40.0 mmol/L.
The most recent survey, which was conducted in Italy [19], reveals no greater consensus; high critical values for platelet count showed quite remarkable disparity for example; values ranged from 449×109/L (barely above most laboratories' reference range) to 1500×109/L.
The variability in interpretation of what constitutes a critical test as well as the variability in the critical limits applied to those tests reflect a serious lack of scientific data to support evidence-based application of the critical-value concept.
In highlighting this Catrou [8] has suggested that critical values might be defined as "those that have been associated with a 90 % probability of death within 24 hours if left untreated".
No such data is available, although there have been some published studies [22-24] aimed at justification of the calcium and sodium critical values used at particular institutions.
The controversy that continues to surround the validity of chosen critical values [10] is reflected in the variable frequency of critical-value reporting. Howanitz et al [12] found that this ranged from 1 in 957 (~ 0.1 %) of all tests reported at some laboratories to 1 in 120 (~ 1 %) of all tests reported, at others.
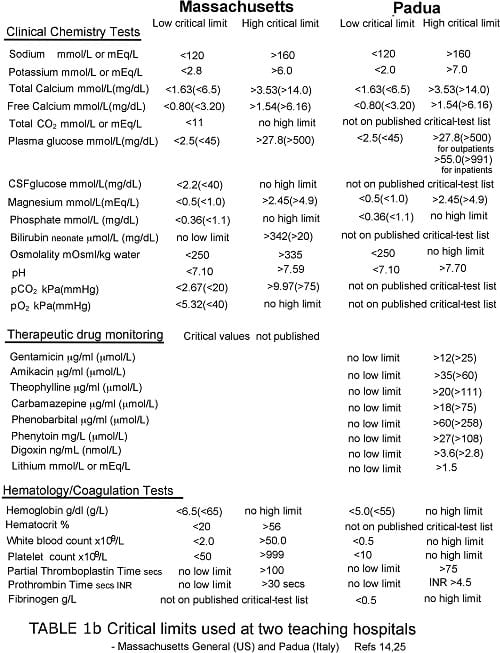
Tables Ia and Ib provide some examples of published critical-test lists and associated critical limits. With paucity of other data, such lists represent an important (arguably the most valid) literature-based resource for guiding the choice of critical values.
REPORTING CRITICAL VALUES
At nearly all (90 %) US healthcare institutions the medical technologist performing the test is responsible for communicating critical values [12,17]. That is not necessarily the case in other parts of the world.
In Italy, for example, senior laboratory staff (medical and scientific) take on this responsibility at most (81 %) hospitals [20]. In common with other authorities [7] including JCHO, Emancipator [5] advises that critical values should ideally be reported directly to the physician caring for the patient.
He acknowledges that this may not always be possible and other staff grades (e.g. nurses and non-clinical staff) identified in the laboratory policy may be recipients of critical values.
The telephone remains the most common mode of communicating critical values.
Howanitz et al [12] found that in US hospitals these calls were made to a variety of staff, including registered nurses (38 % of all calls), non-registered nurses (18.3 %) and ward clerk/office staff (32.5 %).
Their study indicated that the doctor requesting the test was called only rarely (8.6 % of all calls).
Two more recent studies [16,17] suggest that doctors are called more frequently than indicated by Howanitz, but still close to 20 % of US institutions allow communication of critical values to non-clinical staff such as ward clerks or office staff.
In Italian hospitals callbacks are made almost exclusively to either doctors or nurses. Here only 3 % of callbacks are made to ward clerks [20].
Howanitz et al found that the mean time spent communicating a single critical-value result is 6 minutes for inpatient results but more than twice that (14 minutes) for outpatient results.
In around 5 % of cases, inability to contact anyone leads to abandonment of call, and non-communication of a critical-value result [12].
In the interests of patient safety, critical-value policy should include a process for investigation of all such failures so that the cause can be identified and appropriate systematic changes instituted [17].
The Joint Commission requires that all critical-value messages be read back by those receiving the call. The effectiveness of this strategy to reduce communication errors has been demonstrated [21].
Dighe et al found that nearly all (91 %) US laboratories now comply with this requirement and document the read-back [17].
Telephoning critical values, particularly those relating to outpatients, can be extremely time-consuming for laboratory staff.
Despite considerable effort, in a small minority of cases the process fails and the result is not communicated.
The particular problem of communicating critical values to primary-care physicians outside of normal office hours is addressed in an advice document from the UK Royal College of Pathologists [28].
Dighe et al [17] found that centralizing calls at single call center is a partial solution adopted by an increasing minority (17.8 %) of US hospitals. Alternative automatic electronic solutions have been investigated and found to be both more effective than telephone communication and compliant with the safety demands of the Joint Commission [25,26].
Laboratory information systems (LIS) and other technology beyond the telephone is currently under-exploited but has potential to improve many aspects of critical-value reporting [7,17], not least full, reliable documentation of every transaction.
A SINGLE INSTITUTION STUDY
Staff at the clinical laboratories of Massachussets General Hospital (MGH), Boston has a particular research interest aimed at improving critical-value policy. A 2006 analysis of critical-value reporting at their hospital [14] thus provides useful information concerning what might be considered good practice in this area.
Of particular note is the limited number of clinical chemistry and hematology tests (21 plus toxicology/TDM) that are considered critical tests at MGH (see Table Ib).
During a 12-month study period, clinical laboratories at MGH reported 14 million test results, of which 5.1 million were results of tests that appear on their critical-test list (Table Ia).
During that same period the clinical chemistry and hematology laboratories reported a combined total of 37,503 critical values, i.e. 0.25 % of total test results and 0.75 % of critical-test results. The analysis focused on the 37,503 critical values.
Clinical chemistry testing accounted for 68.6 % of critical values reported, hematology testing accounting for the remaining 31.4 %. The two most frequently reported critical values were plasma potassium (7,955; 21.2 % of all critical values reported) and activated partial thromboplastin time, APPT (5,467; 14.6 % of all critical values reported).
Platelet count, glucose and blood gas (pH, pCO2, pO2) were the next most frequent, together accounting for a further 29.8 % of all critical values reported. So these six tests account for around two thirds of all critical values reported at MGH.
For nearly all analytes appearing on the critical-test list <1 % of results were critical values. The exceptions were potassium (1.8 %), APPT (3.0 %), TDM (10.8 %), osmolality (4.5 %) and CSF glucose (6.1 %).
The majority (74 %) of critical values reported were for inpatients, of which half were intensive care patients; 16.9 % were for outpatients and 9.1 % were for emergency room patients.
The laboratory information system (LIS) at MGH automatically flags critical values and laboratory staff (technologists in clinical chemistry and laboratory assistants in hematology) telephone these values to the relevant patient location.
Here, the value is relayed to a "responsible caregiver" (doctor or nurse). The timeliness of this critical-value reporting process was examined. The mean time from identification of a critical value to that value being received at the patient's location was 22 minutes (median 9 minutes).
(A longer time taken for the communication of outpatient results skewed the mean from the median time.) Communication of the result from ward clerk to "responsible caregiver" (doctor or nurse) took on average another 1-2 minutes.
AT MGH an acceptable time frame for reporting critical values has been set at not more than 30 minutes – a target endorsed by others [7,18].
Active investigation of any occasions in which this time frame is not met has reduced the frequency of delays at MGH.
For each critical analyte, result value was plotted against the frequency of that value over the study period. This enabled examination of the potential effect that changing critical limits would have on call volumes.
For example, the plot for glucose showed that by reducing the low critical limit from <3.3 mmol/L (60 mg/dL) to <2.5 mmol/L (45 mg/dL), the number of critical calls could be reduced by 2,136/year (i.e. 5.7 % of all callbacks).
With agreement of clinicians this change, which was judged to not threaten patient safety, was implemented.
REDUCING FALSE POSITIVE CRITICAL VALUES
An important component of critical-care policy is to reduce the number of false positive critical values reported. Reassessment of the critical-test list may reveal tests that are not in fact critical. As in the MGH example cited above, reassessment of critical values may be productive.
Clearly MGH staff concluded that a significant proportion of the critical glucose values they were reporting were not in fact critical at all.
Preanalytical error can lead to false positive critical values.
For example, staff at MGH found that the process being used to transport samples for potassium analysis from outpatients to their laboratory was associated with risk of spurious hyperkalemia [17].
After correcting this preanalytical error (samples were being sent iced rather than maintained at ambient temperature) laboratory staff noted a sustained 20-40 % reduction in the number of critical potassium values (i.e. >6.0 mmol/L) that needed reporting to outpatient locations.
Some critical values are not necessarily critical for all patient groups.
For example, serum creatinine appears on the critical-test list at many hospitals because a markedly raised level can indicate rapidly evolving and potentially life-threatening acute renal failure that may demand urgent dialysis.
However, serum creatinine may not be a critical test for patients with chronic kidney disease presenting for routine dialysis, because for these patients a markedly raised creatinine in excess of critical limits is expected [8,14].
A critical-value policy that allows discrimination between patient groups can significantly reduce the number of critical values that need reporting without threatening patient safety [25].
The capacity to perform delta checks on critical-test results enables the policy of only communicating initial critical values urgently if that is judged acceptable to clinical staff.
MEDICO-LEGAL IMPLICATIONS
According to Dighe and Lewandrowski [27] failure to communicate critical values or to adhere to critical-value policy is an increasingly frequent cause of lawsuits brought against US laboratories and hospitals. They describe two such cases.
The first concerns a 54-year-old male who attended an outpatient phlebotomy center late in the afternoon for routine prothrombin testing to monitor his warfarin therapy.
The result, which became available early that evening, was grossly abnormal (34.8 seconds) indicating over-coagulation and high risk of spontaneous bleeding. In line with critical-value policy at the laboratory, the technologist immediately telephoned the patient's physician with the result.
Unfortunately the physician was unavailable (he was out of town for a few days) so a message was left on his answering service.
In addition, the technologist contacted the customer service department at the hospital for follow-up the next morning. This proved fruitless and the report was mailed to the physician.
In the meantime the patient suffered a serious bleed the day after his blood was tested that resulted in permanent neurological deficit. He successfully sued the laboratory for negligence in that it failed to properly communicate his critical-test result.
The basis of the prosecution was that the laboratory did not have an acceptable policy for communicating critical values in a timely way to an alternative responsible caregiver when the patient's physician is unavailable.
The second case concerns an infant who died from complications associated with diabetic ketoacidosis. The patient first presented several days before her death at her primary-care provider's office.
At this initial visit blood was sampled for blood glucose and sent to the hospital laboratory. Blood glucose was elevated – 302 mg/dL, 16.8 mmol/L.
The written critical-care policy at the laboratory stated that the upper critical limit for glucose was 250 mg/dL (13.9 mmol/L) so the infant's result was by definition a critical value that should have been telephoned immediately.
In fact staff at the laboratory had unofficially adopted an upper critical limit for glucose of 500 mg/dL (27.5 mmo/L) that is more in line with upper critical limit at most hospitals (see Table I).
Since the patient's blood glucose was less than 500 mg/dL, it was not telephoned.
The hospital laboratory was sued for failure to notify the clinician of the "critically" high blood glucose, and because the laboratory had failed to implement its own written policy, the hospital had no option but to settle out of court in favor of the infant's family.SUMMARY
Although laboratories around the world have embraced the concept of critical values since the 1980s, practice in this area has until recently been inconsistent and generally lacking in rigor.Apart from a list of critical values prepared often without much thought or justification, many (perhaps most) clinical laboratories paid little attention to the detail of critical-value policy until a decade ago.
This has changed dramatically with insistence of regulatory authorities that timely critical-value reporting is essential for patient safety and an important component of the overall quality of the clinical laboratory.
Refining the critical-test list and improving the process of critical-value reporting are intellectual and logistical challenges that depend crucially on the continuous cooperative effort of laboratory and clinical staff, and imaginative use of technology.
References+ View more
- Lundberg G. When to panic over abnormal values. Med Lab Observer 1972; 4: 47-54.
- Lundberg G. Critical (panic) value notification: an established laboratory practice policy (parameter). JAMA 1990; 263: 709.
- Centers for Medicare and Medicaid Services. Medicare, Medicaid and CLIA programs: regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA) - HFCA final rule with comment period. Fed Regis 1992; 57: 7002-7186.
- Joint Commission on Accreditation of Healthcare Organizations. 2010 National Patient Safety Goals. Available at:www.jointcommission.org/NR/rdonlyres/6447E5C3-E9EF-4431-885A-7FCF624DD73A/0/July2010NPSGs_Scoring_LAB.pdf. Accessed October 2010.
- Emancipator K. Critical values ASCP practice parameter. Am J Clin Path 1997; 108: 247-53.
- Lippi G, Caputo M, Banfi G et al. Raccomandazioni per l'indentificazione e la gestione dei valori critici nei laboratori clinici. Biochimica Clinica 2008; 32: 209-16.
- Jackson C, MacDonald M, Anderson M et al. Improving communication of critical test results in a pediatric academic setting: key lesson in achieving and sustaining positive outcomes. Healthcare Quarterly 2009; 12: 116-22.
- Catrou P. How critical are critical values? Am J Clin Path 1997; 108: 245-46.
- Plebani M, Lippi G. Improving the post-analytical phase. Clin Chem Lab Med 2010; 48: 435-36.
- Valenstein P. Critical communication. Clin Chem 2010; 56: 334-35.
- Kost G. Critical limits for urgent clinician notification at US medical centers. JAMA 1990; 263: 704-07.
- Howanitz P, Steindel S, Heard N. Laboratory critical values policies and procedures. Arch Pathol Lab Med 2002; 126: 663-69.
- Tilman J, Barth J. A survey of laboratory 'critical (alert) limits' in the UK. Ann Clin Biochem 2003; 40: 181-84.
- Dighe A, Rao A, Coakley A. Analysis of laboratory critical value reporting at a large academic medical center. Am J Clin Path 2006; 125: 758-64.
- Wager E, Stankovic A, Wilkinson D. Assessment monitoring of laboratory critical values. Arch Pathol Lab Med 2007; 131: 44-49.
- Wager E, Friedburg R, Souers R. Critical values comparison. Arch Pathol Lab Med 2007; 131: 1769-75.
- Dighe A, Jones J, Parham S. Survey of critical value reporting and reduction of false positive critical value reports. Arch Pathol Lab Med 2008; 132: 1666-71.
- Valenstein P, Wager E, Stankovic A et al. Notification of critical results. Arch Pathol Lab Med 2008; 132: 1862-67.
- Lippi G, Giavarina D, Montagnana M et al. National survey on critical value reporting in a cohort of Italian laboratories. Clin Chem Lab Med 2007; 45: 1411-13.
- Piva E, Sciacovelli L, Laposta M. Assessment of critical values policies in Italian institutions: comparison with the US situation. Clin Chem Lab Med 2010; 48: 461-68.
- Barenfanger J, Sautter R, Lang D et al. Improving patient safety by repeating (read-back) telephone reports of critical information. Am J Clin Path 2004; 121: 801-03.
- Lum G. Evaluation of a laboratory critical limit (alert value) policy for hypercalcemia. Arch Pathol Lab Med 1996; 120: 633-36.
- Howanitz J, Howanitz P. Evaluation of total serum calcium critical values. Arch Pathol Lab Med 2006; 130: 828-30.
- owanitz J, Howantiz P. Evaluation of serum and whole blood sodium critical values. Am J Clin Pathol 2007; 127: 56-59.
- Piva E, Sciacovelli L, Zaninotto M et al. Evaluation of effectiveness of a computerized notification system for reporting critical values. Am J Clin Pathol 2009; 131: 432-41.
- Parl FF, O'Leary MF, Kaiser AB et al. Implementation of a closed-loop reporting system for critical values and clinical communication in compliance with goals of the Joint Commission. Clin Chem 2010; 56: 417-23.
- Dighe A, Lewandrowski K. Avoiding errors in clinical laboratory critical value reporting. Legal Medicine 2007: 42-49. Available at: www.askafip.org. Accessed October 2010.
- Royal College of Pathologists. Out-of-hours reporting of markedly abnormal laboratory test result to primary care: Advice to pathologists and those that work in laboratory medicine. 2007 RCPATH Available at: www.rcgp.org.uk/PDF/G025v2-OutOfHours-Nov07.pdf. Accessed October 2010.
References
- Lundberg G. When to panic over abnormal values. Med Lab Observer 1972; 4: 47-54.
- Lundberg G. Critical (panic) value notification: an established laboratory practice policy (parameter). JAMA 1990; 263: 709.
- Centers for Medicare and Medicaid Services. Medicare, Medicaid and CLIA programs: regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA) - HFCA final rule with comment period. Fed Regis 1992; 57: 7002-7186.
- Joint Commission on Accreditation of Healthcare Organizations. 2010 National Patient Safety Goals. Available at:www.jointcommission.org/NR/rdonlyres/6447E5C3-E9EF-4431-885A-7FCF624DD73A/0/July2010NPSGs_Scoring_LAB.pdf. Accessed October 2010.
- Emancipator K. Critical values ASCP practice parameter. Am J Clin Path 1997; 108: 247-53.
- Lippi G, Caputo M, Banfi G et al. Raccomandazioni per l'indentificazione e la gestione dei valori critici nei laboratori clinici. Biochimica Clinica 2008; 32: 209-16.
- Jackson C, MacDonald M, Anderson M et al. Improving communication of critical test results in a pediatric academic setting: key lesson in achieving and sustaining positive outcomes. Healthcare Quarterly 2009; 12: 116-22.
- Catrou P. How critical are critical values? Am J Clin Path 1997; 108: 245-46.
- Plebani M, Lippi G. Improving the post-analytical phase. Clin Chem Lab Med 2010; 48: 435-36.
- Valenstein P. Critical communication. Clin Chem 2010; 56: 334-35.
- Kost G. Critical limits for urgent clinician notification at US medical centers. JAMA 1990; 263: 704-07.
- Howanitz P, Steindel S, Heard N. Laboratory critical values policies and procedures. Arch Pathol Lab Med 2002; 126: 663-69.
- Tilman J, Barth J. A survey of laboratory 'critical (alert) limits' in the UK. Ann Clin Biochem 2003; 40: 181-84.
- Dighe A, Rao A, Coakley A. Analysis of laboratory critical value reporting at a large academic medical center. Am J Clin Path 2006; 125: 758-64.
- Wager E, Stankovic A, Wilkinson D. Assessment monitoring of laboratory critical values. Arch Pathol Lab Med 2007; 131: 44-49.
- Wager E, Friedburg R, Souers R. Critical values comparison. Arch Pathol Lab Med 2007; 131: 1769-75.
- Dighe A, Jones J, Parham S. Survey of critical value reporting and reduction of false positive critical value reports. Arch Pathol Lab Med 2008; 132: 1666-71.
- Valenstein P, Wager E, Stankovic A et al. Notification of critical results. Arch Pathol Lab Med 2008; 132: 1862-67.
- Lippi G, Giavarina D, Montagnana M et al. National survey on critical value reporting in a cohort of Italian laboratories. Clin Chem Lab Med 2007; 45: 1411-13.
- Piva E, Sciacovelli L, Laposta M. Assessment of critical values policies in Italian institutions: comparison with the US situation. Clin Chem Lab Med 2010; 48: 461-68.
- Barenfanger J, Sautter R, Lang D et al. Improving patient safety by repeating (read-back) telephone reports of critical information. Am J Clin Path 2004; 121: 801-03.
- Lum G. Evaluation of a laboratory critical limit (alert value) policy for hypercalcemia. Arch Pathol Lab Med 1996; 120: 633-36.
- Howanitz J, Howanitz P. Evaluation of total serum calcium critical values. Arch Pathol Lab Med 2006; 130: 828-30.
- owanitz J, Howantiz P. Evaluation of serum and whole blood sodium critical values. Am J Clin Pathol 2007; 127: 56-59.
- Piva E, Sciacovelli L, Zaninotto M et al. Evaluation of effectiveness of a computerized notification system for reporting critical values. Am J Clin Pathol 2009; 131: 432-41.
- Parl FF, O'Leary MF, Kaiser AB et al. Implementation of a closed-loop reporting system for critical values and clinical communication in compliance with goals of the Joint Commission. Clin Chem 2010; 56: 417-23.
- Dighe A, Lewandrowski K. Avoiding errors in clinical laboratory critical value reporting. Legal Medicine 2007: 42-49. Available at: www.askafip.org. Accessed October 2010.
- Royal College of Pathologists. Out-of-hours reporting of markedly abnormal laboratory test result to primary care: Advice to pathologists and those that work in laboratory medicine. 2007 RCPATH Available at: www.rcgp.org.uk/PDF/G025v2-OutOfHours-Nov07.pdf. Accessed October 2010.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar








