Printed from acutecaretesting.org
July 2012
Effect of small air bubbles on changes in blood pO2 and blood gas parameters: calculated vs. measured effects
It is well known that air bubbles erroneously introduced into blood collected in syringes can affect blood gas results, especially the pO2 value [1,2]. This pO2 interference is greater if the air bubble is vigorously mixed with the blood, such as by intense shaking [2] or by pneumatic tube transport [3].
We wanted to quantitate by both calculations and measurements how increments of smaller air bubbles (20 and 40 µL) added and equilibrated into blood can change pO2 over a range of initial pO2 values.
It is simple to calculate the O2 content of 10 to 100 µL air bubbles at atmospheric pressure (Table I), and relatively easy to calculate the O2 content of an air bubble at various pO2 values (Table II for 20 µL and 40 µL air bubbles). However, it is more challenging to estimate the effect this increased O2 content would have on the pO2 value of a blood sample for several reasons:
-
The amount of O2 that can be transferred from air to blood depends on the difference in initial pO2 between blood and the air bubble.
-
After equilibrium is achieved between air and blood, the only assumption that can be made is that pO2 will be the same in air and blood. However, the pO2 of both air and blood would change, and could either increase or decrease, depending on the initial pO2 of blood (assuming an atmospheric pO2).
-
Assuming an initial pO2 in air of 150 mmHg, the pO2 of blood would usually increase (for persons breathing atmospheric air), but could decrease (for persons breathing oxygen-enriched air), or change very little (if blood has an initial pO2 of approximately 150 mmHg). This is further complicated because blood is typically exposed to air bubbles at room temperatures (RT; 21-24 °C), but is analyzed in a closed system at 37 °C. Thus, for blood at pO2 150 mmHg (37 °C), exposure to air at 150 mmHg (RT) should increase the pO2 in blood to above 150 mmHg.
-
The cooler room temperature relative to 37 °C will also increase the affinity of Hb for O2. This will increase the transfer of O2 from air to blood with pO2 < 150 mmHg.
-
The measurement of pO2 detects the oxygen dissolved in the aqueous phase of blood and not the larger reservoir of oxygen bound to Hb. Oxygen bound to Hb is not detected when pO2 is measured, although Hb-O2 does contribute to the equilibrium of O2 in the aqueous phase of blood.
-
Depending on the initial saturation of Hb (%O2Hb), the change in pO2 is not linear as oxygen is added to blood. This is because Hb can bind some of the O2 absorbed from the air bubble, depending on the initial saturation of Hb with oxygen. For example, at a low sO2 (i.e., 60 %), when O2 is added to blood, more oxygen will bind to Hb, with relatively less change in pO2. If Hb is nearly saturated with oxygen (i.e., 98 %), more of the added oxygen dissolves in the aqueous phase of blood, causing a larger change in pO2.
-
The concentration of Hb also adds to the complexity. For examples, at an initial pO2 of 90 mmHg and an Hb of 6 g/dL, an increase in O2 content of 0.5 mL O2/dL blood will increase pO2 from 90 to about 170 mmHg. At Hb = 15 g/dL, the pO2 will increase from 90 to only 120 mmHg.
Given these challenges, we sought to calculate the expected theoretical change in pO2 when either 20 µL or 40 µL increments of air are added to blood.
To determine the validity of these calculations, we measured blood gas and CO-oximetry parameters on 19 blood samples to which we added 20 and 40 µL increments of atmospheric air and equilibrated this air with the blood. We also calculated the measured changes in pH and pCO2 of blood after adding these increments of air.
METHODS AND CALCULATIONS
Measurements
We measured blood gases and CO-oximetry parameters with two different standard blood gas/CO-oximetry analyzers. The most relevant measurements for this study were: pO2, Hb, sO2, %O2Hb, O2 content and calculated sO2 (from pO2). pH and pCO2 were also measured.
Calculations
The calculated O2 content of air bubbles (10 to 100 µL) at atmospheric pressure is shown in Table I and the calculated O2 content of an air bubble at various pO2 values is shown in Table II for 20 µL and 40 µL air bubbles.
| Bubble vol (µL) | O2 content (mL O2/dL blood) |
O2 content (mL O2/mL blood) |
| 10 | 0.21 | 0.0021 |
| 20 | 0.42 | 0.0042 |
| 30 | 0.63 | 0.0063 |
| 50 | 1.05 | 0.0105 |
| 100 | 2.09 | 0.0209 |
TABLE I: O2 content of 10-100 µL air bubbles; assuming an atmospheric gas pressure of 710 mmHg = 760 – 47 (47 mmHg is the contribution of water vapor to atmospheric pressure).
| pO2 (mmHg) | O2 content of 20 µL air bubble (mL O2) |
O2 content of 40 µL air bubble (mL O2) |
| 40 | 0.00113 | 0.00225 |
| 50 | 0.00141 | 0.00282 |
| 60 | 0.00169 | 0.00338 |
| 70 | 0.0020 | 0.0040 |
| 80 | 0.00225 | 0.00451 |
| 85 | 0.0024 | 0.0048 |
| 90 | 0.00253 | 0.00507 |
| 95 | 0.0027 | 0.0054 |
| 100 | 0.00282 | 0.00563 |
| 110 | 0.0031 | 0.0062 |
| 120 | 0.00338 | 0.00676 |
| 150 | 0.00423 | 0.00845 |
| 200 | 0.00563 | 0.01127 |
| 250 | 0.00704 | 0.01408 |
| 300 | 0.00845 | 0.01690 |
| 400 | 0.0113 | 0.0226 |
TABLE II: O2 content vs. pO2 for 20 and 40 µL air bubbles at atmospheric gas pressure of 710 mmHg (760 – 47).
To estimate how the change in O content would affect O of blood, several parameters were first calculated. The O was calculated for various values of O using the Severinghaus equation [4] with the aid of an online calculator [5]. With this data and using the equation for O content of blood, the O content was calculated vs. O for Hb levels, with the calculations for 10 g/dL Hb shown in Table III.
| pO2 (mmHg) | sO2 (% O2Hb) | O2 content (mL O2/dL blood) |
O2 content (mL O2/mL blood) |
| 20 | 32.0 | 4.414 | 0.0441 |
| 30 | 57.4 | 7.899 | 0.0790 |
| 40 | 74.9 | 10.310 | 0.1031 |
| 50 | 85.0 | 11.715 | 0.1172 |
| 60 | 90.6 | 12.508 | 0.1251 |
| 70 | 93.8 | 12.974 | 0.1297 |
| 80 | 95.7 | 13.263 | 0.1326 |
| 90 | 96.9 | 13.457 | 0.1346 |
| 100 | 97.7 | 13.597 | 0.1360 |
| 110 | 98.3 | 13.710 | 0.1371 |
| 120 | 98.7 | 13.795 | 0.1380 |
| 150 | 99.3 | 13.970 | 0.1397 |
| 200 | 99.7 | 14.179 | 0.1418 |
| 250 | 99.9 | 14.361 | 0.1436 |
| 300 | 99.9 | 14.516 | 0.1452 |
| 400 | 100.0 | 14.840 | 0.1484 |
TABLE III: Calculation of O2 content vs. pO2 in blood with [Hb] = 10 g/dL.
Although we initially calculated this with an online Arterial Oxygen Content Calculator [6], we had to manually calculate the O2 content to 3 decimal places because small changes in O2 content have a large effect on pO2. The equation used in the calculation was:
O2 content (mL/dL blood) = 1.36 × Hb × sO2 + 0.0031 × pO2;
with Hb in g/dL; sO2 as decimal percent; and pO2 in mmHg.
Using an Hb of 10 g/dL as an example, we calculated the O2 content of blood at pO2s from 20 to 250 mmHg (Table IV). However, we had to estimate the percent of oxygen that would be extracted from both a 20 µL (Table IVa) and a 40 µL air bubble (Table IVb).
We reasoned that blood at a low pO2, such as 40 mmHg, would extract more O2 from the bubble (~80 %) than if the pO2 were 100 mmHg (~32 %). For blood at pO2 higher than 150 mmHg, the O2 would transfer from blood into the air bubble. However, the amount transferred would be limited because the pO2 of the air and blood must both remain above 150 mmHg.
Therefore, if blood at a pO2 of 250 mmHg has 0.1436 mL O2/mL blood (Table III and IV) and if the O2 content can go no lower than 0.1397 mL O2/mL blood (O2 content of blood at 150 mmHg), then the maximum amount of O2 that could be lost from blood is 0.0039 mL/mL blood (0.1436 – 0.1397).
From this, we calculated the estimated change in O2 content, the new O2 content of blood, the new pO2 of blood, and finally the change in blood pO2 at each initial pO2 of blood, which are shown in the last four columns of Tables IVa and IVb.
| Initial pO2 (mmHg) | Initial O2 contenta | Assumed % extraction of O2 from bubble | Estimated change in O2 contenta | New O2 content of blooda | Estimated New pO2 (mmHg)c | Change in blood pO2 (mmHg) |
| 20 | 0.0441 | 90 | 0.00378 | 0.0479 | 21 | +1 |
| 40 | 0.1031 | 80 | 0.00336 | 0.1065 | 42 | +2 |
| 60 | 0.1251 | 60 | 0.00252 | 0.1276 | 66 | +6 |
| 80 | 0.1326 | 45 | 0.00189 | 0.1345 | 90 | +10 |
| 90 | 0.1346 | 40 | 0.00168 | 0.1363 | 103 | +13 |
| 100 | 0.1360 | 32 | 0.00134 | 0.1373 | 113 | +13 |
| 120 | 0.1380 | 20 | 0.00084 | 0.1388 | 135 | +15 |
| 150 | 0.1397 | 10 | 0.00042 | 0.1401 | 160 | +10 |
| 200 | 0.1418 | -10 | -0.0004 b | 0.1414 | 190 | -10 |
| 250 | 0.1436 | -30 | -0.0012 b | 0.1424 | 215 | -35 |
TABLE IVa: Estimates of pO2 changes when 20 µL air bubble is added to blood with Hb = 10 g/dL.
From Table I, a 20 µL air bubble contains 0.0042 mL O2/mL blood.
a Units for O2 content are mL O2/mL of blood.
b For blood at pO2 250 mmHg, the maximum volume of O2 that can be lost from blood to an air bubble is 0.0039 mL O2/mL blood (0.1436 – 0.1397), since the blood pO2 must stay above 150 mmHg (from Table III).
c The pO2 was estimated from the O2 content vs. pO2 data in Table III.
| Initial pO2 (mmHg) | Initial O2 contenta | Assumed % extraction of O2 from bubble | Estimated change in O2 contenta | New O2 content of blooda | Estimated New pO2 (mmHg)c | Change in blood pO2 (mmHg) |
| 20 | 0.0441 | 90 | 0.00756 | 0.0517 | 22 | +2 |
| 40 | 0.1031 | 80 | 0.00672 | 0.1098 | 44 | +4 |
| 60 | 0.1251 | 60 | 0.00504 | 0.1301 | 72 | +12 |
| 80 | 0.1326 | 45 | 0.00378 | 0.1364 | 104 | +24 |
| 90 | 0.1346 | 40 | 0.00336 | 0.1380 | 120 | +30 |
| 100 | 0.1360 | 32 | 0.00269 | 0.1387 | 132 | +32 |
| 120 | 0.1380 | 20 | 0.00168 | 0.1397 | 150 | +30 |
| 150 | 0.1397 | 10 | 0.00084 | 0.1405 | 171 | +21 |
| 200 | 0.1418 | -15 | -0.0006 b | 0.1412 | 185 | -15 |
| 250 | 0.1436 | -40 | -0.0016 b | 0.1420 | 205 | -45 |
TABLE IVb: Estimates of pO2 changes when 40 µL air bubble is added to blood with Hb = 10 g/dL.
From Table I, a 40 µL air bubble contains 0.0084 mL O2/mL blood.
a Units for O2 content are mL O2/mL of blood.
b For a blood pO2 of 250 mmHg, the maximum volume of O2 that can be lost from blood into an air bubble is 0.0039 mL O2/mL blood (0.1436 – 0.1397), since the blood pO2 must stay above 150 mmHg (from Table III).
c The pO2 was estimated from the O2 content vs. pO2 data in Table III.
Preparation of samples
Using approximately 1 to 1.6 mL of blood from 19 discarded de-identified heparinized blood samples after blood gas analysis, we removed all visible air bubbles, mixed by rotation, then analyzed on blood gas analyzers for blood gas parameters, including pH, pCO2, pO2, total Hb, %O2Hb, sO2 and O2 content.
Following this, we successively added 20 µL or 40 µL of atmospheric air to the sample, with thorough equilibration of blood and air before analysis. Thus, we obtained data with either 40, 80, 120, 160 µL, etc. air added, or 20, 40, 60, 80 µL, etc. air added to each sample.
The volume of air added was approximated by visually pulling back on the syringe plunger until the syringe tip was either half full of air (~20 µL) or full of air (~40 µL). While this will not give a highly accurate volume of air, it has the advantage of minimally exposing the blood to extraneous air. Samples were analyzed on either of two different blood gas analyzers, with six on one analyzer and 13 on the other analyzer.
RESULTS AND CONCLUSIONS
While the data in Table IV shows the theoretical changes in pO2 as increments of air were added to blood, to determine if our calculations are valid, we measured blood gas and CO-oximetry parameters on 19 blood samples before and after various volumes of air were added and mixed into the blood.Blood gas/CO-oximetry results from a representative blood sample with 40 µL increments of air added are shown in Table V, which also includes the calculated difference in pO2 results (ΔpO2) as each increment of air was added.
Fig. 1 shows both the theoretical changes in pO2 as either 20 or 40 µL air bubbles are added to blood and the actual changes in pO2 measured in the 19 blood samples. The measured changes for pO2 in Fig. 1 confirm the general trends of the calculated changes. The other conclusions from this figure follow.
| Time | 10:20 | 10:24 | 10:28 | 10:32 | 10:36 | 10:40 |
| Bubble vol (µL) | 0 | 40 | 80 | 120 | 160 | 200 |
| pH | 7.243 | 7.241 | 7.241 | 7.244 | 7.248 | 7.255 |
| pCO2 (mmHg) | 34.5 | 34.1 | 33.7 | 32.9 | 32.0 | 30.9 |
| pO2 (mmHg) | 69.4 | 85.1 | 105 | 127 | 159 | 183 |
| ΔpO2 (mmHg) | 16 | 20 | 22 | 32 | 24 | ---- |
| Hb (g/dL) | 13.7 | 13.6 | 13.5 | 13.6 | 13.6 | 13.6 |
| %O2Hb | 89.7 | 93.3 | 95.0 | 95.8 | 96.1 | 96.2 |
| sO2 % | 92.6 | 96.2 | 97.8 | 98.8 | 99.1 | 99.3 |
| O2 ct (mL/mL) | 0.173 | 0.179 | 0.182 | 0.185 | 0.187 | 0.187 |
TABLE V: Blood gas and CO-oximetry measurements on a representative arterial blood sample as 40 µL increments of room air were added to 1.2 mL blood. The ΔpO2 is the difference between the pO2 values before and after addition of each 40 µL increment of air. O2 ct = oxygen content.
-
Adding air has very different effects on the pO2 of blood, depending on the initial pO2 of the blood. Adding air has minimal effect at low pO2 values (20-40 mmHg), and moderate effects up to about 60 mmHg. At 80-150 mmHg, the increase in pO2 can be quite large, ranging from 20 to 30 mmHg.
We also note that, despite blood at low initial pO2 extracting more O2 from the air bubbles, the changes in blood pO2 were much less at lower initial pO2 values. This is because Hb is less saturated and has a greater capacity to bind oxygen, such that less oxygen is added to the aqueous phase of blood, which determines the pO2.
In effect, if Hb is about 90 % or less saturated with oxygen, it is able to buffer the increase in pO2 when air is added. At saturations of 95 % and above, Hb apparently has less ability to buffer changes in pO2 if air is added to blood. -
Although we added oxygen in air at atmospheric pressure (pO2 of ~150 mmHg), the amount of O2 absorbed by blood will depend on the difference between 150 mmHg and the actual pO2 of blood. Thus, for blood at a low pO2 (i.e., 60 mmHg), more O2 from the gas bubble would be absorbed.
For blood at 150 mmHg, essentially no O2 would be absorbed by the blood (assuming no temperature effects), and for blood at high pO2, O2 would be lost from the blood into the air bubble. Being unaware of an equation to calculate the percent of O2 absorbed by blood from air at differing pO2 values, we estimated such percentages.
In Tables IVa and IVb, we show the percent of O2 in 20 and 40 µL air bubbles that would be absorbed by blood at pO2 values ranging from 20 to 250 mmHg. For example, in blood at pO2 40 mmHg, we estimate that a large proportion of O2 in the air bubble would be absorbed by blood (~80 %) at an Hb concentration of 10 g/dL.
At a pO2 of 250 mmHg, oxygen would move from blood into the air bubble. Furthermore, since the blood pO2 could not go below 150 mmHg, we estimated that the maximum O2 lost from blood into the air bubble would be 0.0039 mL/mL blood (0.1436 – 0.1397 from Table III).
With these assumptions, we could then calculate the change in O2 content, the new O2 content of blood, and (using data from Table III) the new pO2 of blood (Table IV). The last column in Tables IVa and IVb shows the change in blood pO2 for the initial pO2 of blood.
-
Because of the aforementioned temperature effects, adding air has almost no effect on blood with an initial pO2 of 170-190 mmHg. It became clear that adding air to blood could increase pO2 to well above 150 mmHg, typically to 170-180 mmHg.
This was readily explained because the equilibration with the air bubble was done at room temperature (approx. 22 °C), while the analysis was at 37 °C. At cooler temperatures, Hb increases its affinity for O2 and O2 becomes more soluble in the aqueous phase of blood.
So while more O2 would be absorbed by blood at the cooler room temperature, this O2 would be released during analysis at 37 °C. After slightly modifying the data in Table IV to account for this effect, we plotted the theoretical changes in pO2 vs. the initial pO2 when either 20 or 40 µL increments of oxygen were added (Fig. 1), along with the pO2 data from individual blood samples.
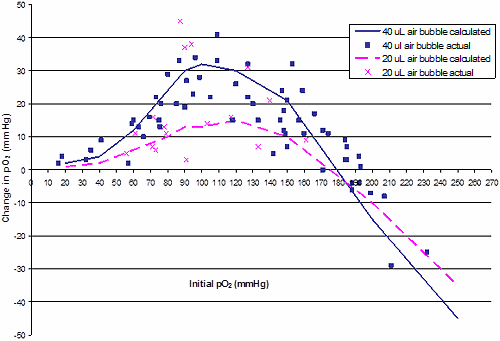
FIG. 1: Calculated and measured changes in blood pO2 when 20 or 40 µL air (atmospheric pO2) was added to blood. Data points are based on changes in pO2 as measured on 19 blood specimens as air was sequentially introduced and equilibrated with the blood in a syringe.
The results in this study confirm the changes reported previously in pO2 values during pneumatic transport caused by air contamination of blood gas specimens [3].
| Our results suggest that for air bubbles of 20 µL or less, the effect on pO2 is less than 10 mmHg up to about 70 mmHg. At initial pO2 values of 80 to 140 mmHg, 20 µL air can have a wide range of effects, ranging from 5 to 40 mmHg. In fact, at initial pO2 values of 90 mmHg, 20 µL air bubbles often had as much effect as 40 µL air bubbles. |
Since the Hb level should affect these changes, we calculated the theoretical changes at different Hb levels of 8, 10 and 12 g/dL. However, the effect was fairly minimal (data not shown).
| Air volume added | |||
| 40 µL | 80 µL | 120 µL | |
| Mean ΔpH | 0.004 | 0.006 | 0.014 |
| min | –0.01 | –0.01 | 0.00 |
| max | 0.04 | 0.04 | 0.05 |
| Mean Δ pCO2 | –0.76 | –1.41 | –2.84 |
| min | –3.0 | –4.0 | –9.0 |
| max | 1.0 | 0.2 | –0.9 |
TABLE VI: Mean changes in pH and pCO2 in the 19 blood samples tested as 40 µL increments of room air were added to blood. The mean initial pH of the samples was 7.32 (range 6.89-7.53) and the initial pCO2 of the samples was 47.9 mmHg (range 31-84 mmHg).
The changes in pH and pCO2 were more straightforward. As air was added, changes to these parameters were small compared to changes in pO2. The mean change in pH was +0.02 for 120 µL air added. For pCO2, the mean change was –1 mmHg for 40 µL air added and about –3 mmHg for 120 µL air added (see Table VI). Clearly, air has much less effect on the pH and pCO2 than on the pO2.
References+ View more
Madiedo G, Sciacca R, Hause L. Air bubbles and temperature effect on blood gas analysis. J Clin Pathol 1980; 33: 864-67.
Biswas CK, Ramos JM, Agroyannis B, Kerr DNS. Blood gas analysis: Effect of air bubbles in syringes and delay in estimation. Brit Med J 1982.
Astles JR, Lubarsky D, Loun B, Sedor FA, Toffaletti J. Pneumatic transport exacerbates interference of room air contamination in blood gas samples. Arch Pathol Lab Med 1996; 120: 642-47.
Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol 1979; 46: 599-602.
http://www-users.med.cornell.edu/~spon/picu/o2satcal.htm. Website accessed Jan 2012.
http://www.globalrph.com/arterial_oxygen_content.htm. Website accessed Jan 2012.
Collinson PO, John CM, Gaze DC, Ferrigan LF, Cramp DG. Changes in blood gas samples produced by a pneumatic tube system. J Clin Pathol 2002; 55: 105-07.
References
Madiedo G, Sciacca R, Hause L. Air bubbles and temperature effect on blood gas analysis. J Clin Pathol 1980; 33: 864-67.
Biswas CK, Ramos JM, Agroyannis B, Kerr DNS. Blood gas analysis: Effect of air bubbles in syringes and delay in estimation. Brit Med J 1982.
Astles JR, Lubarsky D, Loun B, Sedor FA, Toffaletti J. Pneumatic transport exacerbates interference of room air contamination in blood gas samples. Arch Pathol Lab Med 1996; 120: 642-47.
Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol 1979; 46: 599-602.
http://www-users.med.cornell.edu/~spon/picu/o2satcal.htm. Website accessed Jan 2012.
http://www.globalrph.com/arterial_oxygen_content.htm. Website accessed Jan 2012.
Collinson PO, John CM, Gaze DC, Ferrigan LF, Cramp DG. Changes in blood gas samples produced by a pneumatic tube system. J Clin Pathol 2002; 55: 105-07.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Minimizing Pre-analytical errors in blood gas testing
Presented by Ana-Maria Simundic, PhD, Prof. of Medical Biochemistry, Zagreb University, Zagreb, Croatia Watch the webinarRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar