Printed from acutecaretesting.org
April 2012
Why measure blood gases? A three-part introduction for the novice. Part 2.
Reference (normal) range for the blood gas parameters under discussion here:
pH 7.35-7.45
pCO2(a) 4.7-6.0 kPa (35-45 mmHg)
Bicarbonate 22-28 mmol/L
DISTURBANCE OF ACID-BASE HOMEOSTASIS - GENERAL CONSIDERATION
Disturbance of acid-base homeostasis is characterized by abnormality in one or more of three parameters (pH, pCO2(a) and bicarbonate) generated during blood gas analysis. So long as all three parameters are within their respective reference (normal) range, it can be assumed that the mechanisms involved in maintaining the pH of blood within healthy limits (i.e. acid-base homeostasis) are working adequately and normal acid-base status is assured.Nearly all clinically significant disturbances of acid-base homeostasis can be attributed to one or more of three broad causes:
- disease of, or damage to, any one of four organs (kidney, lungs, brain, gastrointestinal tract) whose normal function, as outlined in the first article of this series, is necessary for acid-base homeostasis.
- disease that results in abnormally increased production of metabolic acids, such that normal acid-base homeostatic mechanisms outlined in the first article of this series are overwhelmed.
- medical intervention (e.g. artificial ventilation and a number of drugs can cause or contribute to acid-base disturbance).
Given the wide range of medical conditions that can be associated with disturbance of acid-base balance it is useful, when presented with a patient whose acid-base is disturbed, to narrow down the possible cause by classifying that particular patient’s acid-base disturbance to one of four classes, which are:
- respiratory acidosis
- respiratory alkalosis
- metabolic acidosis
- metabolic alkalosis
CLASSIFICATION OF ACID-BASE DISTURBANCE
To understand how patient ABG results (pH, pCO2(a) and bicarbonate concentration) are used to identify an acid-base disturbance and, through classification, narrow down its cause, we must return to a relationship between these three measured parameters that was introduced in the first article:
pH α [HCO3-] Note: [HCO3– ] = bicarbonate concentration pCO2(a)
This simple relationship, which is crucial to an understanding of all that follows, reveals that the pH of blood is a function of both bicarbonate concentration and pCO2(a), specifically that pH is proportional to the ratio of bicarbonate concentration to pCO2(a). Importantly, for understanding patient ABG results, the relationship allows the following five deductions:
1 - Blood pH remains normal so long as the ratio [HCO3–] : pCO2 remains normal.
2 - Blood pH increases (i.e. alkalosis occurs) if either [HCO3–] increases or pCO2(a) decreases.
3 - Blood pH decreases (i.e. acidosis occurs) if either [HCO3–] decreases or pCO2(a) increases.
4 - If both pCO2(a) and [HCO3–] are increased by relatively the same amount, the ratio and therefore blood pH are normal.
5 - If both pCO2(a) and [HCO3–] are decreased by relatively the same amount, the ratio and therefore blood pH are normal.
The two components, pCO2(a) and bicarbonate concentration, that determine blood pH in the above relationship are referred to as the ”respiratory” and ”metabolic” (or non-respiratory) components, respectively, when discussing acid-base balance.
This nomenclature reflects the physiological truth, as outlined in the previous article, that the primary determinant of pCO2(a) is respiratory rate (ventilation), and a primary determinant of bicarbonate concentration is the amount of metabolic acid added to blood.
The primary abnormality in those with respiratory acidosis/alkalosis is to pCO2(a) and the primary abnormality in those with metabolic acidosis/alkalosis is to bicarbonate concentration [HCO3–]. Thus,RESPIRATORY ACIDOSIS is characterized by increased pCO2(a), which in line with deduction 3 above results in reduced pH (i.e. acidosis).
RESPIRATORY ALKALOSIS is characterized by decreased pCO2(a), which in line with deduction 2 above results in increased pH (i.e. alkalosis).
METABOLIC ACIDOSIS is characterized by decreased bicarbonate, which in line with deduction 3 above results in decreased pH (i.e. acidosis).
METABOLIC ALKALOSIS is characterized by increased bicarbonate, which in line with deduction 2 above results in increased pH (i.e. alkalosis).
CAUSES OF THE FOUR ACID-BASE DISTURBANCES
Respiratory acidosis (primary increase in pCO2(a), reduced pH)
Respiratory acidosis, defined by increased pCO2(a), is almost invariably the result of inadequate alveolar ventilation (hypoventilation). In effect, accumulation of CO2 in blood is due to reduced excretion by the lungs.
A number of pulmonary (lung) conditions can be associated with hypoventilation sufficient to cause respiratory acidosis, including chronic obstructive airways disease (COAD), acute respiratory distress syndrome (ARDS), bronchopneumonia, severe ( life-threatening) acute asthma and pulmonary edema. Disease or trauma to the chest wall and the musculature involved in the mechanics of respiration can result in hypoventilation.
This explains the respiratory acidosis that can occur in poliomyelitis, Guillain-Barré syndrome and traumatic chest injury (flail chest).
Some drugs (barbiturates and opiates, e.g. morphine), as well as head injury can result in respiratory acidosis by depressing or damaging the respiratory center in the brain that regulates the respiratory rate. Respiratory acidosis may be acute (i.e. of rapid onset, as in acute asthma attack) or chronic (i.e. long-standing, as in COAD between acute exacerbation). Respiratory failure is defined by pCO2(a) >6.5 kPa.
RESPIRATORY ALKALOSIS (PRIMARY DECREASE IN pCO2(a) INCREASED pH)
Respiratory alkalosis, defined by decreased pCO2(a), is always the result of increased alveolar ventilation (hyperventilation). In effect, excessive rate of breathing leads to increased excretion of CO2 by the lungs and consequent reduced amount of CO2 in blood.Stimulation of the respiratory center in the brain and consequent hyperventilation is the cause of the respiratory alkalosis that is often a feature of anxiety (panic) attacks and response to severe pain or other major stressor. The increased ventilation that is provoked by asthma attack can result in respiratory alkalosis. Increased ventilation is a normal physiological response to reduced oxygen in blood (hypoxemia).
Hypoxemia-induced respiratory alkalosis might be evident in patients with severe anemia, those at high altitude where oxygen tension of inspired air is reduced, and patients with respiratory disease associated with severe hypoxemia such as acute respiratory distress syndrome (ARDS) Cushui disease. Salicylate in overdose stimulates the respiratory center, causing respiratory alkalosis. Excessive artificial ventilation has the same effect.
METABOLIC ACIDOSIS (PRIMARY DECREASE IN BICARBONATE, DECREASED pH)
Reduced bicarbonate is the defining feature of all cases of metabolic acidosis and occurs for one of three reasons. Firstly, bicarbonate can be consumed in buffering an abnormally high acid load, so the primary problem here is increased production of metabolic acids. The second reason is increased loss of bicarbonate from the body and the third reason is failure of kidneys to regenerate bicarbonate.
In a critical care setting metabolic acidosis is the most frequent acid-base disturbance and the most common cause is increased production of the metabolic acid, lactic acid. Lactic acid is produced in excess by tissue cells that are poorly oxygenated, so metabolic (lactic) acidosis can arise in any clinical condition in which oxygen delivery to tissues is compromised.
Examples of such critical conditions include hypoxemia, severe anemia, reduced cardiac output and resulting poor tissue perfusion (e.g. cardiac arrest, hypovolemic shock due to, for example, severe hemorrhage or burns) and sepsis.
The liver is the major site of lactic acid metabolism so that accumulation of lactic acid and resulting metabolic acidosis can arise in advanced liver disease (cirrhosis, liver failure).
Increased production of the metabolic acids, ß-hydroxybutryric acid and acetoacetic acid (collectively called ketoacids) is the cause of the metabolic acidosis that results from insulin deficiency in patients with diabetes. This life-threatening acute complication of diabetes is called diabetic ketoacidosis. A similar metabolic disturbance called alcoholic ketoacidosis can occur with excessive alcohol intake (binge drinking).
In both lactic acidosis and ketoacidosis bicarbonate is consumed in buffering excess acid.
Increased loss of bicarbonate via the gastrointestinal tract is the cause of the metabolic acidosis the can occur with protracted diarrhea, vomiting of bile (rich in bicarbonate), and in patients with pancreatic fistula. Reduced bicarbonate regeneration by the kidneys and reduced urinary excretion of hydrogen ions contribute to the metabolic acidosis evident in acute and chronic renal failure.
Finally, salicylate overdose is associated with increased production of several metabolic acids. Since it can also result in concomitant respiratory alkalosis (see above), salicylate overdose represents a clinical situation that can give rise to what is called a mixed acid-base disturbance, to be discussed later.
METABOLIC ALKALOSIS (PRIMARY INCREASE IN BICARBONATE, INCREASED PH)
The increased bicarbonate concentration that characterizes metabolic alkalosis is most commonly due to abnormal loss of hydrogen ions (acid) from the body. Thus, loss of gastric acid accounts for the metabolic alkalosis that results from the vomiting of gastric contents associated with pyloric stenosis. Aspiration of gastric contents has the same effect.
Increased loss of hydrogen ions in urine due to excessive secretion of glucocorticoid hormones explains the increased bicarbonate and resulting metabolic alkalosis that can be a feature of Cushing’s disease. A similar mechanism associated with excessive mineralocorticoid hormone accounts for the metabolic alkalosis that occurs in patients with Conn’s syndrome.
Excessive IV bicarbonate administration or ingestion of bicarbonate in antacid preparations can potentially cause metabolic alkalosis but this is usually transient because the kidneys have the capacity to increase bicarbonate excretion, if necessary.
In those with renal dysfunction, however, this may not be the case as evidenced by so called milk-alkali syndrome, which is caused by excessive ingestion of bicarbonate containing antacid tablets, and characterized by the triad: metabolic alkalosis, renal dysfunction and hypercalcemia.
Metabolic alkalosis is common in patients with severe hypokalemia. This is due in large part to the movement of hydrogen ions from ECF into cells in exchange for potassium ions; a movement that restores ECF potassium at the expense of increased ECF pH.
Increased reabsorption of bicarbonate is a side effect of some diuretic drugs (frusemide, thiazide) that can be sufficient to cause metabolic alkalosis in a minority of patients.
PHYSIOLOGICAL CONSEQUENCE OF ACID-BASE DISTURBANCE - COMPENSATION
All acid-base disturbances are associated with a tendency to either reduced blood pH (acidosis) or increased blood pH (alkalosis). Because of the prime importance of maintaining blood pH within the reference (normal) range, acid-base disturbances provoke physiological responses aimed at normalizing blood pH.
These physiological responses, collectively referred to as compensation, are reflected in blood gas results and partly explains the counterintuitive notion - alluded to at the top of this article - that patients with an acid-base disturbance may have a normal blood pH.
To understand the process of compensation and the way it affects blood gas results, it is important to recall that the pH of blood is governed by the ratio of bicarbonate concentration to pCO2(a) and that this relationship allows deductions 1, 4 and 5 above. In the case of respiratory disturbances in which pH is abnormal because pCO2(a) is abnormal, the compensatory response is to make an equivalent change to bicarbonate concentration, thereby normalizing the ratio, and therefore pH.
By contrast, metabolic disturbances, which are always due to abnormality in bicarbonate, are compensated for by an equivalent change in pCO2(a).
To illustrate in a little more detail the process of compensation, consider the patient who is suffering diabetic ketoacidosis. This patient is suffering a metabolic acidosis due to an accumulation in blood of ketoacids. As bicarbonate is being consumed in buffering this excess acid, bicarbonate concentration and, eventually, blood pH fall. In order to compensate for the reduction in bicarbonate concentration and return the all-important ratio (bicarbonate : pCO2(a)) towards normal, it is necessary for pCO2(a) to also be reduced.
This is accomplished by increasing the rate of CO2 excretion by the lungs; i.e. increasing respiratory ventilation. In all cases of metabolic acidosis, including diabetic ketoacidosis, chemoreceptors in the brain respond to the rising concentration of hydrogen ions (decreasing pH), causing an increased rate of ventilation. This results in increased elimination of CO2, reduced pCO2(a) and thereby restoration of the all-important ratio (bicarbonate : pCO2(a)) towards normal.
The deep, labored, sighing respiration (called Kussmaul respiration), which is evident in patients suffering diabetic ketoacidosis, is symptomatic evidence of respiratory compensation for the metabolic (keto) acidosis.
The compensation for primary respiratory disturbances depends on renal mechanisms that regulate bicarbonate concentration. Thus compensation for respiratory acidosis involves increased renal reabsorption of bicarbonate to blood, and compensation for respiratory alkalosis involves decreased renal reabsorption of bicarbonate and thereby decreased blood bicarbonate concentration.
Renal compensation of primary respiratory acid-base disturbances is, by comparison with respiratory compensation of primary metabolic acid-base disturbance, a relatively slow process occurring over a period of several days. The presence or absence of a compensatory response distinguishes acute (no evidence of compensation) from chronic respiratory disturbances (compensation evident).
By contrast, respiratory compensation of primary metabolic disturbances is rapid, beginning within minutes and complete within 12-24 hours. It is thus rare for there to be no evidence of compensation in primary metabolic disturbances.
Figure 1 provides a visual representation of pH, bicarbonate and pCO2(a) during all four acid-base disturbances, before and after full compensation; it reflects the notion, contained in the relationship above, that blood pH is a ”balance” between bicarbonate concentration and pCO2(a).
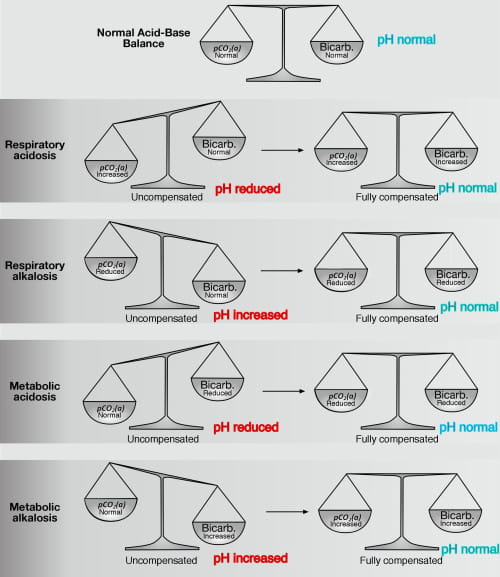
FIG1: The "acid-base" balance: pH pCO2(a) and bicarbonate values before and after full compensation. Note: Compensation restores normal pH
Respiratory compensation of metabolic alkalosis is somewhat limited by the hypoxemic potential of hypoventilation (the compensatory physiological response to metabolic alkalosis). It is evidently physiologically more vital to maintain oxygen delivery to tissues than to maintain normal blood pH.
Whatever the primary acid-base disturbance or secondary compensatory response, a patient is said to be fully compensated if blood pH is within the normal range, and partially compensated if blood pH has returned towards normal, but not actually achieved normality.
In practice, full compensation is uncommon and overcompensation is physiologically impossible. Thus even with maximally compensated acidosis (either respiratory or metabolic) pH usually remains less than 7.35 (and is never greater than 7.40). By the same token maximally compensated alkalosis (either respiratory or metabolic) is usually associated with pH greater than 7.45 (and never with pH less than 7.40).
Although compensation may not achieve normal pH, it is very common for pH to return very close to normality as a result of maximal compensation.
The finding of a normal blood pH in a patient with acid-base disturbance is less likely to be due to full compensation of a single acid-base disorder and more likely to be due to the combined effect (alkalosis plus acidosis) of a mixed acid-base disorder.
MIXED ACID-BASE DISORDERS
Thus far it has been assumed that patients with acid-base disturbance suffer just one of the four classes of acid-base disturbance discussed above. Whilst that is indeed most often the case, a significant minority of patients with acid-base disturbance - perhaps around 20-30 % - present with a mixture of two, or rarely, three classes of disturbance.
As an example of a mixed acid-base disorder, consider a patient with severe COAD who has a heart attack and suffers cardiac arrest. Before the heart attack the patient has a partially, or more rarely, fully compensated respiratory acidosis due to long-standing COAD. During arrest his blood gas results reflect the combined effect of compensated respiratory acidosis due to COAD and metabolic acidosis due to inadequate tissue perfusion and consequent lactic acidosis.
Given the range of clinical conditions and drug toxicities that cause each of the single acid-base disorders, it is not difficult to imagine many other clinical situations in which patients might be suffering more than one class of acid-base disturbance.
Salicylate poisoning is notable as a single condition that can give rise to a mixed acid-base disorder (respiratory alkalosis due to depression of respiration and metabolic acidosis due to excess (exogenous) acid). In conditions like salicylate poisoning in which a tendency to both acidosis and alkalosis occur together, the two disturbances may cancel each other out, resulting in normal blood pH.
As might be suspected, blood gas results are much more difficult to interpret in the context of mixed acid-base disturbances than in the context of a single acid-base disorder.
Indeed, they really cannot be interpreted without consideration of patient’s clinical condition and drug history. Single acid-base disorders, by contrast, can usually be identified by reference to blood gas results (pH, pCO2(a), bicarbonate) alone; Fig. 2 provides an algorithm for the diagnosis of single acid-base disorders (with or without compensation). Table I summarizes the most common causes of acid-base disturbance.
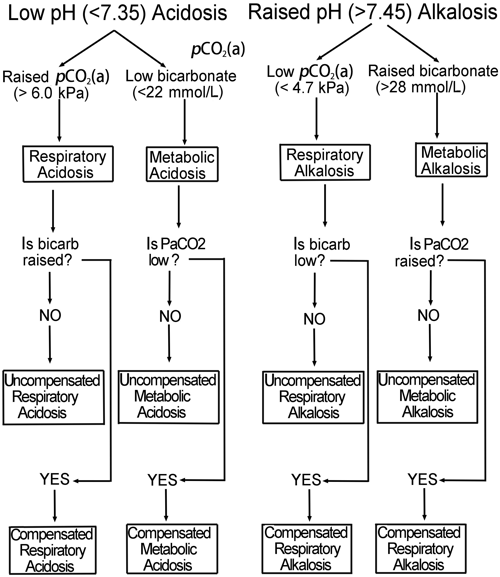
FIG2: Algorithim for diagnosing acid-base disturbance from pH, pCO2(a) and bicarbonate

TABLE I: Causes of single acid-base disturbance and examples of clinical situations associated with mixed disturbance
BULLET-POINT SUMMARY
- The blood gas parameters used to assess acid-base status are pH, pCO2(a) and bicarbonate.
- If all three are within their respective reference range, normal acid-base status is assured.
- Disturbance of acid-base status can be caused by a wide range of diseases, some drugs and other medical interventions, most notably artificial ventilation.
- Acid-base disturbances are classified, using pH, pCO2(a) and bicarbonate results, to one of four types: metabolic acidosis, metabolic alkalosis, respiratory acidosis and respiratory alkalosis. This classification is useful in identifying the cause and therefore the most appropriate treatment.
- Whilst most patients with acid-base disturbance suffer only one of the four types, some have mixed acid-base disturbance, making interpretation of blood gas results more complex.
- Acid-base disturbances provoke a physiological response, referred to as compensation, that aims to return abnormal pH towards normal. This compensatory response is evident in blood gas results.
SUGGESTED LEARNING RESOURCES
- West J. Respiratory physiology - the essentials. 8th ed. Baltimore: Lippincott Williams & Wilkins, 2008
- Dominiczak M, Szczepanska-Konkel M. Regulation of hydrogen ion concentration (acid-base balance) In: Medical Biochemistry. 3rd ed. CBS: City of publication mangler: Mosby, 2009.
- Waugh A, Grant A. The respiratory system. In: Ross and Wilson anatomy and physiology in health and illness. 11th ed. CBS: City of publication mangler: Churchill-Livingstone, 2010.
- Hennesey I, Japp A. Arterial blood gases made easy. CBS: City of publication mangler: Churchill-Livingstone, 2007.
References+ View more
- West J. Respiratory physiology - the essentials. 8th ed. Baltimore: Lippincott Williams & Wilkins, 2008.
- Dominiczak M, Szczepanska-Konkel M. Regulation of hydrogen ion concentration (acid-base balance) In: Medical Biochemistry. 3rd ed. Mosby, 2009.
- Waugh A, Grant A. The respiratory system. In: Ross and Wilson anatomy and physiology in health and illness. 11th ed. Churchill-Livingstone, 2010.
- Hennesey I, Japp A. Arterial blood gases made easy. Churchill-Livingstone, 2007.
References
- West J. Respiratory physiology - the essentials. 8th ed. Baltimore: Lippincott Williams & Wilkins, 2008.
- Dominiczak M, Szczepanska-Konkel M. Regulation of hydrogen ion concentration (acid-base balance) In: Medical Biochemistry. 3rd ed. Mosby, 2009.
- Waugh A, Grant A. The respiratory system. In: Ross and Wilson anatomy and physiology in health and illness. 11th ed. Churchill-Livingstone, 2010.
- Hennesey I, Japp A. Arterial blood gases made easy. Churchill-Livingstone, 2007.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









