Printed from acutecaretesting.org
July 2004
Effective POCT: the need for a broader perspective
Introduction
In recent years, the focus on developments in point-of-care testing (POCT) have moved away from a preoccupation with the analytical process to a broader perspective that takes into consideration other procedures which take place before and after the measurement process.
This has come about through a realization that success with POCT requires optimization of the many different steps that make up the total testing process or cycle (Fig. 1).
Outside forces have also played a role in this change of focus, particularly those concerned with promoting total quality management and patient safety such as the International Standardization Organization (ISO), the Institute of Quality and national accreditation agencies [1].
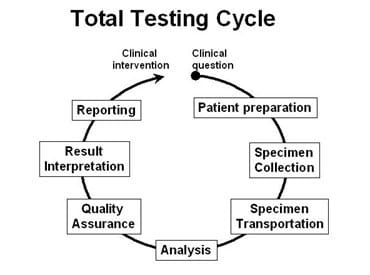
Fig. 1.
The organization and management of the whole POCT process can be considered as two layers. The first layer comprises the steps that are taken on a day-to-day basis to ensure that every time a sample is analyzed, appropriate quality procedures are adhered to and monitored. These procedures are largely the ones identified in Figure 1 and relate to pre- and postanalytical processes.
This day-to-day management operates within the framework of an institution’s POCT policy. This is determined by the second layer of organization and management and includes matters such as which laboratory tests should be performed by POCT as well as processes for equipment procurement, training and audit.
Day-to-day management of POCT
Figure 1 gives an indication of the many potential steps that need to be completed correctly in order to obtain a valid result from a POCT device. The number of steps will depend to some extent on the nature of the analyte and the location of the device.
Yet the complexity of, and potential for, preanalytical mistakes should never be underestimated, so documented procedures together with good training are essential.
Preanalytical processes also extend or overlap with the analytical phase in the sense that several key processes need to take place at the instrument but before the actual analysis takes place. The importance of good instrument design was mentioned earlier and this is crucial in relation to the user interface.
While much is made of miniaturization in terms of the ability to make smaller devices, an equally important trend has been the introduction of hardware from the consumer electronics market, such as touch screens into benchtop and now even smaller POCT devices.
Instruments being provided with barcode readers are another important feature, and the dividends in terms of improving compliance with preanalytical processes are well described [2].
In effect, manufacturers have had such an impact here in recent years that overall design and ease-of-use features provide an increasingly important way to differentiate POCT devices that are otherwise similar in terms of test menu and analytical performance.
A recurring theme when discussing pre- and postanalytical processes in relation to instrument design is the importance of informatics on POCT [3]. It is now nearly three years since the CIC Connectivity standard was adopted by the industry and we are now starting to see the benefits as manufacturers introduce the POCT-1A standard into their devices [4].
The result will be an increasing number of software features that provide solutions to pre- or postanalytical problems.
For example, POCT test results are generated simultaneously with test orders, unlike in the general laboratory where the request is generated in advance of the test, and the information flow through the Laboratory Information System (LIS) allows a requisition/sample number to be generated in advance of the sample being presented to an instrument for processing.
Now POCT devices solve this problem in a number of ways, one of which is a two-stage data interchange in which a pending request is made by the POCT system to obtain a requisition number which is then transmitted back to the LIS with the result (Figure 2). Clearly connectivity to the LIS or other information systems is vital to this process.
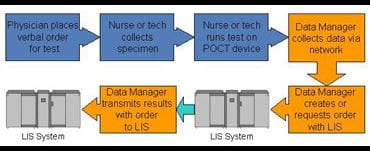
Fig. 2.
The daily organization and management of POCT has also benefited enormously from other information technology advancements including the now widespread availability of IT networks.
The latter has facilitated the development of software with critical care testing analyzers which allows the central laboratory to remotely monitor and control devices which may be long distances from the point of control.
This reduces the need to physically visit each device which can result in significant staff savings and therefore improve the cost effectiveness of POCT [5]. While these packages have generally been proprietary, and primarily in the area of blood gases and related parameters, the adoption of POCT-1A is now leading to more flexible systems which enable the interfacing of a wider range of other devices.
Postanalytical processes that are vital to patient care include the need to ensure that patient and quality-related data is directed into a permanent record; this issue was a major driving force for the adoption of a connectivity software standard.
This need becomes more important as more and more institutions move to electronic records, and the value of these will be significantly reduced if they do not include all patient data, including that generated at point-of-care.
There are a variety of other destinations for point-of-care data, including bedside monitors and clinical information systems that reside in critical care units.
These systems integrate data from various sources, including vital signs as well as diagnostic results, and in conjunction with clinical guidelines and expert systems can produce critical care maps to be used in the management of the critically ill patient [6].
Institution-wide management of POCT
Behind the day-to-day procedures associated with managing POCT lies another tier of policy and decision management that is also critical to the success of POCT. POCT involves many different parties outside of the laboratory and the need to consult widely is shown in the worldwide trend of appointing POCT Coordinating Committees within hospital and other medical institutions.
The membership of these committees will vary between places, but laboratory and nursing staff are key members together with representatives of departments who are requiring POCT services. Guiding the work of the committee will be the institution’s policy on POCT and this will be one based on the principles of total quality management and the increasingly important issue of clinical governance [7].
The latter is about ensuring accountability of staff involved with the service and that patients’ interests are protected. The aim of the policy is to provide a high-quality, cost-effective POCT service.
One of the key tasks of the committee will be to review requests for a POCT service. This may not be an easy process for a test that has previously been performed in the central laboratory but is now requested to be performed at point-of-care. Some of the questions that need to be asked in dealing with any such request are shown in Table I.
They will include the clinical necessity for a POCT service, which analytes should be measured and the outcomes that should result from what is a change in laboratory practice.
In the past, many of these questions have been avoided and this has led to conflicts between laboratories and clinical departments about the pros and cons of POCT, and making objective decisions in this area is one important purpose of the POCT Coordinating Committee.
Having decided to proceed with a POCT service for a particular analyte or group of analytes, one of the first management tasks will be to provide the best equipment or device for the purpose.
Prior to evaluating equipment, the required analytical performance, turnaround time and skill levels of the likely users should be determined.
One key issue in equipment evaluation is determining the level of agreement between the POCT device and results for the same analyte in the central laboratory; in some cases there can be substantial differences or biases and this will require constant monitoring by those responsible for the POCT service.
Also important is to evaluate the performance of the instrument in the hands of those who will be using it on a day-to-day basis and not in the hands of laboratory-trained staff whose performance will nearly always be better [8].
While the suppliers of equipment will provide training as part of the purchase and installation process, an important responsibility of POCT management is to provide regular training both for new and existing staff. Furthermore, there is an increasing need to assess the competency of staff as part of these training programs.
Currently and particularly in those countries where accreditation of all POCT devices does not exist, it remains a significant challenge to get all staff performing POCT to adhere to all the required quality procedures.On occasions, busy caregivers will always find what they believe is a legitimate reason for not carrying out the correct quality process.
Thus in several countries including Australia, clinical scientist organizations are developing links with nursing education groups with the purpose of developing a curriculum around POCT that is taught to nurses as an integral part of obtaining their qualifications.
This will be an important step towards achieving a better level of understanding of concepts such as quality assurance by those staff who are carrying out POCT and should contribute to better compliance with required practice.
In addition, manufacturers are progressively including features, primarily software-related, which either automate quality control sampling procedures or demand that the operator performs them at certain times before a patient sample can be analyzed.
Preparation of documented procedures, sometimes referred to as standard operating procedures, is an integral part of the total quality management of POCT and forms part of all standard accreditation procedures. Documentation includes a host of responsibilities including not only the patient result, but also all quality data, maintenance procedures and records of training.
Once again the designers of POCT devices have helped with many of these tasks in recent years with the provision of memories on all but the smallest of devices and substantial databases on larger instruments. These together with ease of connectivity to other information systems are enabling the recording of all relevant data.
A final and increasingly important task in relation to providing a high-quality POCT service is to review its actual performance on a regular basis. Audit procedures for POCT are about ensuring that it is meeting the needs for which it was intended, and in effect this means going back to addressing some of the questions that were asked when the service was initially established (Table I).
Some parts of the audit are relatively easy to implement, such as checking that quality assurance procedures are being conducted. Difficult questions include whether the most effective clinical outcomes are being achieved from the POCT service.
There has been much discussion in the literature concerning the difficulties of determining outcomes, but studies are now appearing which have shown both the positive and unchanged outcomes that can be achieved from POCT [9].
What has emerged from several of these studies is that to obtain the benefits of POCT it may be necessary to make more fundamental changes to the way that the testing service is delivered, and just placing a device closer to the patient and improving the turnaround time may not be sufficient [10].
In the future, the quality of POCT will continue to be a partnership between the manufacturers and suppliers of devices and clinical scientists managing the service.
The former will continue to remove many of the potential problems of actually using the device through better design and user-friendly features, as they continue on the path of striving to design a foolproof device.
Meanwhile, clinical scientists and other laboratory staff will concentrate on ensuring that the patient data gets to the right location and optimizing the whole POCT process to ensure that it delivers the best benefits to the caregiver and patient.
|
TABLE I. Issues to be addressed by a POCT Coordinating Committee
References+ View more
- Auxter S. Partnering with others to ensure patient safety. Clin Lab News 2003; 29: 1-4.
- Bernard D. Laboratory supervision of point-of-care blood gas testing. Accessed April 12, 2004
- Jones R. St John A. Informatics and point-of-care testing. In: Price CP, St John A, Hicks JM, eds. Point-of-Care Testing. 2nd Ed. AACC Press, 2004; 197-207.
- National Council for Clinical Laboratory Standards: Point-of-Care Connectivity; Approved Standard. NCCLS Document POCT1-A. Wayne, PA. National Council for Clinical Laboratory Standards 2001
- Hirst D, St John A. Keeping the spotlight on quality from a distance. Accred Qual Assur 2000; 5: 9-13
- Halpern N. Point of care diagnostics and networks. Crit Care Clin 2000; 16: 623-39
- Freedman DB. Clinical governance – bridging management and clinical approaches to quality in the UK. Clin Chim Acta. 2002; 319: 133-41
- Skeie S, Thue G, Nerhus K, Sandberg S. Instruments for self-monitoring of blood glucose: comparisons of testing quality achieved by patients and a technician. Clin Chem 2002; 48: 994-1003.
- St John A, Price CP. Measures of outcome. In: Price CP, Christenson RH (eds). Evidence-Based Laboratory Medicine. From Principles to Outcomes. Washington: AACC Press, 2003: 55-74.
- Nichols JH, Kickler TS, Dyer KL, Humbertson SK, Cooper PC, Maughan WL, Oechsle DG. Clinical outcomes of point-of-care testing in the interventional radiology and invasive cardiology setting. Clin Chem 2000; 46: 543-50
References
- Auxter S. Partnering with others to ensure patient safety. Clin Lab News 2003; 29: 1-4.
- Bernard D. Laboratory supervision of point-of-care blood gas testing. Accessed April 12, 2004
- Jones R. St John A. Informatics and point-of-care testing. In: Price CP, St John A, Hicks JM, eds. Point-of-Care Testing. 2nd Ed. AACC Press, 2004; 197-207.
- National Council for Clinical Laboratory Standards: Point-of-Care Connectivity; Approved Standard. NCCLS Document POCT1-A. Wayne, PA. National Council for Clinical Laboratory Standards 2001
- Hirst D, St John A. Keeping the spotlight on quality from a distance. Accred Qual Assur 2000; 5: 9-13
- Halpern N. Point of care diagnostics and networks. Crit Care Clin 2000; 16: 623-39
- Freedman DB. Clinical governance – bridging management and clinical approaches to quality in the UK. Clin Chim Acta. 2002; 319: 133-41
- Skeie S, Thue G, Nerhus K, Sandberg S. Instruments for self-monitoring of blood glucose: comparisons of testing quality achieved by patients and a technician. Clin Chem 2002; 48: 994-1003.
- St John A, Price CP. Measures of outcome. In: Price CP, Christenson RH (eds). Evidence-Based Laboratory Medicine. From Principles to Outcomes. Washington: AACC Press, 2003: 55-74.
- Nichols JH, Kickler TS, Dyer KL, Humbertson SK, Cooper PC, Maughan WL, Oechsle DG. Clinical outcomes of point-of-care testing in the interventional radiology and invasive cardiology setting. Clin Chem 2000; 46: 543-50
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








