Printed from acutecaretesting.org
September 2004
Hematocrit - a review of different analytical methods
INTRODUCTION
This article describes the advantages and disadvantages of the different methods of measuring hematocrit by discussing the following:
- The components of whole blood
- Indications for measuring hematocrit
- Measuring technologies
1. Microhematocrit
2. Complete blood cell count
3. Conductivity on blood gas analyzers
4. Calculation of hematocrit - Comparison of the different technologies
This article will be followed by another article describing the different methods of determining hemoglobin.
THE COMPONENTS OF WHOLE BLOOD
Whole blood is comprised of erythrocytes (the red blood cells or RBCs involved in oxygen transport), platelets and leukocytes (the white blood cells or WBCs involved in the body's immune defense). The cells are suspended in the aqueous medium of plasma.
In blood from healthy individuals, erythrocytes constitute the vast majority of cells; the erythrocytes contain hemoglobin (Hb), which gives blood its red color and which has oxygen-binding abilities. Plasma mainly consists of water (approx. 93 %) but also of salts, various proteins and lipids as well as other constituents, e.g. glucose.
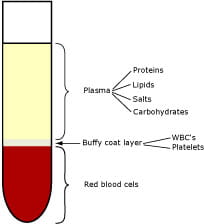
FIG. 1. A centrifugated whole-blood sample
The definition of hematocrit (hemato from the Greek haima = blood; crit from the Greek krinein = to separate) is the ratio of the volume of packed red blood cells to the total blood volume and is therefore also known as the packed cell volume, or PCV.
The hematocrit is reported as a percentage or a ratio. In healthy adult individuals the red blood cells constitute approx. 40-48 %, whereas newborns may have hematocrits of up to 60 % [1].
The layer between the RBCs and plasma, the buffy coat layer, constitutes approx. 1 %. It consists of WBCs and platelets and should therefore not be calculated as part of the packed cell volume.
The relationship between hematocrit and hemoglobin
The following is a summary of the quantities/abbreviations that are relevant when discussing hematocrit:
- Hct: Hematocrit (% or volume fraction)
- ctHb: Concentration of total hemoglobin (g/dL, g/L or mmol/L)
- RBC: Red blood cell (erythrocyte) (× 1012/L)
- MCV: Mean cell volume (fL)
- MCHC: Mean corpuscular hemoglobin concentration (%, g/L or mmol/L)
In normal conditions there is a linear relationship between hematocrit and the concentration of hemoglobin (ctHb). An empirical study [2] has shown that the relationship can be expressed as follows:
|
Hct (%) = (0.0485 × ctHb (mmol/L) + 0.0083) × 100 |
Hematocrit can also be estimated from measurements of the mean cell volume (MCV) or the mean corpuscular hemoglobin concentration (MCHC):
|
Hct (%) = MCV × RBC × 0.1 |
|
Hct (%) =
ctHb
× 100
|
INDICATIONS FOR MEASURING HEMATOCRIT
Hematocrit measurements may be requested when it is suspected that a patient is anemic or suffering from dehydration, bleeding or other medical and surgical conditions.
Low hematocrit
A low hematocrit reflects a low number of circulating red blood cells and is an indicator of a decrease in the oxygen-carrying capacity or of overhydration. Examples of conditions causing a low hematocrit (anemia) include [3]:
- Internal or external hemorrhage – bleeding
- Complication of chronic renal failure – kidney disease
- Pernicious anemia – vitamin-B12 deficiency
- Hemolysis – associated with transfusion reactions
A low hematocrit may be found in autoimmune diseases and bone-marrow failures.
High hematocrit
A high hematocrit may reflect an absolute increase in the number of erythrocytes, or a decrease in plasma volume, in conditions such as [3]:
- Severe dehydration – e.g. in case of burns, diarrhea or excessive use of diuretics
- Erythrocytosis – excessive red blood cell production
- Polycythemia vera – abnormal increase of blood cells
- Hemachromatosis – an inherited iron metabolism disorder
High hematocrit is also used as an indicator of the excessive intake of exogenous erythropoitin (EPO), which stimulates the production of red blood cells. Athletes can artificially improve their performance by enhancing the oxygen-carrying capacity with EPO.
In newborns and especially premature babies, high hematocrit values are common. The hematocrit of infants reaches the level of adult hematocrit by approx. three months of age [1].
The conditions leading to low hematocrit values, e.g. hemorrhage, often require continuous measurements of the hematocrit and fast decisions concerning transfusions. If the hematocrit is measured immediately after an acute hemorrhage, the value will be normal until the decreased blood volume is corrected by fluid shifts into the blood vessels.
MEASURING TECHNOLOGIES
This section gives a technical description of the most commonly used techniques for measuring hematocrit:
- The determination of hematocrit by means of centrifugation
- The calculation of hematocrit from the complete blood cell count (CBC)
- The determination of hematocrit by conductivity
- The calculation of hematocrit from ctHb
1. Microhematocrit
The reference method recommended by NCCLS of determining hematocrit or packed cell volume (PCV) is centrifugation.
Method [4]:
Hematocrit (PCV) is the measure of the ratio of the
volume occupied by the red blood cells to the volume of whole
blood. The blood sample is drawn into a capillary and
centrifugated, and then the ratio can be measured and expressed
as a decimal or percentage fraction.
Materials:
- Whole blood from a freely flowing skin puncture or anticoagulated (EDTA or heparin) venous or arterial blood
- Glass capillary tubes with a narrow diameter
- Sealing compound (if the capillaries are not self-sealing)
- A microhematocrit centrifuge with a maximum relative centrifugal force of 10-15,000 × g, which should be reached within 30 seconds [4]
- Graphic reading device
Procedure:
- Capillary tubes are filled by capillary forces. A minimum of two capillaries is required to ensure balance in the centrifuge. It is important that the tubes are sealed thoroughly.
- After five minutes of centrifugation the hematocrit can be measured while the tubes are still kept in a horizontal position. A distinct column of packed erythrocytes is visible in one end of the capillary tube (Fig. 2). The packed erythrocytes are followed by first a small turbid layer – the buffy coat layer – and then a clear column of plasma (Fig. 2). Hematocrit is estimated by calculating the ratio of the column of packed erythrocytes to the total length of the sample in the capillary tube, measured with a graphic reading device.
- The measurement should be performed within 10 minutes to avoid merging of the layers.
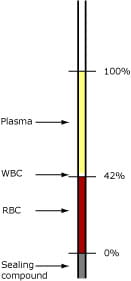
FIG. 2. Reading the hematocrit
Limitations:
- Studies have shown that spun hematocrit gives values approx. 1.5-3.0 % too high due to plasma trapped in the RBC layer. If abnormal types of RBCs are present, this bias can be even greater, as more plasma is trapped [5]. See also Table I.
2. Complete blood cell count (CBC)
In hematology laboratories, automatic cell count analyzers measuring multiple parameters are the most commonly used.
Method:
The hematocrit is determined indirectly from the
average size and number of RBCs. The reference method is the
Coulter impedance principle [6] and is described below.
Materials:
- Sample tubes normally containing 3-5 mL EDTA anticoagulated blood
Procedure:
- The whole-blood sample is diluted automatically with an isotonic solution prior to analysis.
- The diluted blood is forced through an orifice which has two electrodes placed on opposite sides (Fig. 3).
- By applying a constant current between the two electrodes, the impedance is constant until a blood cell passes through the orifice
- Due to the non-conductive properties of the red blood cell membrane, the electrical resistance increases each time an erythrocyte passes through the orifice.
- The change in potential between the electrodes correlates to the volume of the passing erythrocyte. Furthermore, erythrocytes that have passed through the orifice are counted. From the mean cell volume, the erythrocyte count and the dilution factor, the hematocrit is derived.
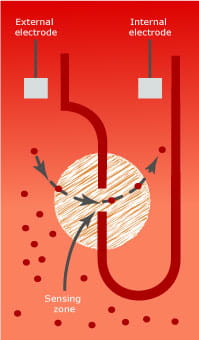
FIG. 3. The Coulter principle
Limitations:
- When a high reticulocyte or WBC count is present, hematocrit determinations using hematology analyzers can result in the calculation of falsely elevated values, because the higher cell volumes of these cells will interfere with the red blood cell count and the calculation of the hematocrit [7]. See also Table I.
3. Conductivity on blood gas analyzers
In POCT, blood gas analyzers measuring multiple STAT parameters are often used. Some blood gas analyzers determine hematocrit by a conductivity measurement which is corrected for the concentrations of conducting ions in the sample.
Materials:
- Syringes or capillaries containing heparinized arterial or venous blood
Method:
- The conductivity is the ability of a solution to transmit (conduct) electricity. The electrical current will increase in proportion to the number of ions (or charged particles) found in a solution, their electrical charge and mobility, i.e. how easily the ions can move in the solution. The mobility of an ion in a solution will also depend on how many cells (and size and shape) are suspended in the solution.
- Both erythrocytes and plasma have characteristic electrophysical properties. The membrane of the erythrocytes is electrically insulating, mainly due to its content of lipids, so that it appears essentially non-conducting.
- Plasma is fairly conductive due to its content of electrolytes and charged proteins; the major contributor to plasma conductivity is Na+, the concentration in human blood plasma being approx. 140 mmol/L.
- Due to this, there is an inverse relationship between the electrical conductance and the hematocrit in blood when the concentration of the charged particles is taken into account.
Three factors besides the number of RBCs are critical for the determination of the hematocrit value when using a method based on measurement of electrical conductivity:
- Electrolytes
- Temperature
- Proteins
Most blood gas analyzers allow for these variables as follows:
- Concentration of electrolytes: This is determined by one or more ion measurements. As sodium is the primary electrolyte in plasma, this is the most important ion to measure and use in the calculation of hematocrit.
- A change in the temperature has a significant impact on the conductivity because blood has a high temperature coefficient. The measuring chamber in blood gas analyzers is thermostatted and the blood sample preheated prior to measurement; thus there is no contribution from changing temperature.
- The protein concentration in plasma is assumed constant in healthy people, so a constant compensation for this is incorporated in the calculation of hematocrit on blood gas analyzers.
Limitations:
- In patients with abnormal plasma osmolality, e.g. patients being treated with plasma expanders, blood diluents or massive infusion therapy, the protein concentration is no longer constant and the hematocrit determination gives falsely low values [8, 9]. Some blood gas analyzers offer correction for this bias [10,11,12]. See also Table I.
4. Calculation of hematocrit from hemoglobin
As there is a linear relationship between hemoglobin (ctHb) and hematocrit as described earlier, it is possible to calculate the hematocrit on analyzers that measure hemoglobin. When making this conversion, two factors should be taken into consideration:
- The analytical quality of the ctHb measurement
- The precision of the equation that converts the two parameters
The measurement of ctHb from most good-quality analyzers is usually reliable; however, the equations used to calculate the hematocrit vary from analyzer to analyzer. Some analyzers use an empirically found equation [2,13] whereas others use an approximate conversion factor of 3 [14,15].
Example:
|
Hb concentration
|
Conversion equation |
Hct % |
Reference |
|
15* |
Hct (%) = (0.0485 × ctHb (mmol/L) + 0.0083) × 100 |
45.98 |
[2] |
|
15 |
Hct (%) = 2.8 × ctHb (g/dL) + 0.8 |
42.80 |
[13] |
|
15 |
Hct (%) = ctHb (g/dL) / 0.34 |
44.12 |
[16] |
|
15 |
Hct (%) = 2.941 × ctHb (g/dL) |
44.12 |
[14,15] |
TABLE I: Effect of different conversion factors on
Hct %.
* Conversion factor: g/dL × 0.62058 = mmol/L
Limitations:
- It is generally assumed that the conversion from hemoglobin to hematocrit is straightforward since most methods measuring ctHb are considered to be fairly accurate; however, different analyzers use different conversion factors, which may compromise the reliability of the hematocrit result. Hematocrit and hemoglobin are often used interchangeably; however, different studies have shown that the two parameters are not comparable, but that they have their separate applications [15,17,18,19].
Comparing measuring technologies
All measuring technologies for determining hematocrit have advantages and disadvantages. The following table provides an easy overview of the described methods. The advantages/disadvantages listed have all been found by reviewing the literature, i.e. no prioritizing according to importance has been done.
|
Method |
Advantages |
|
Microhematocrit |
|
|
Complete blood cell count |
|
|
Conductivity |
|
|
Calculation from ctHb |
|
TABLE IIa. Advantages of different methods of measuring hematocrit.
|
Method |
Disadvantages |
|
Microhematocrit |
|
|
Complete blood cell count |
|
|
Conductivity |
|
|
Calculation from ctHb |
|
TABLE IIb. Disadvantages of different methods of measuring hematocrit.
Discussion
When hematocrit is used to assess anemia and the oxygen-carrying capacity, the advantages and disadvantages of each method must be carefully considered. In addition, the specific clinical and analytical needs for any patient population must be determined.
Hematocrit is traditionally a routine hematology laboratory parameter; however, measuring hematocrit as a STAT parameter in a POC setting is in many ways preferable, as this will save time in critical situations and avoid specimen transport problems. Some blood gas analyzers offer this possibility, either by measuring hematocrit by conductivity or by calculating hematocrit from ctHb.
Hemoglobin is also used to assess anemia both in the laboratory and as a STAT parameter on POCT analyzers. Different hemoglobin measuring methods and their applications will be discussed in another article.
Conclusion
Both POCT and traditional laboratory methods of measuring or calculating hematocrit have limitations. Methods used outside of the laboratory must be intuitive for users without time-consuming sample handling.
Methods suitable for an adult environment may not be suitable for a neonatal environment due to sample volume limitations. Some methods are not suitable in certain applications due to e.g. problems associated with volume expansion fluids or abnormally sized or shaped red blood cells.
These limitations can have important clinical implications and must be carefully considered as described in this article.
References+ View more
- Soldin SJ, Rifai N, Hicks JMB. Biochemical Basis of Pediatric Disease. Second ed. Washington DC: AACC Press, 1995: 5.
- Kokholm G. Simultaneous measurements of blood pH, pCO2, pO2 and concentrations of hemoglobin and its derivatives – a multicenter study. Radiometer publication AS107. Copenhagen: Radiometer Medical A/S, 1991.
- http://www.nlm.nih.gov/medlineplus/encyclopedia.html Medline Plus encyclopedia
- Bull BS, Koepke JA, Simson E et al. Procedure for determining packed cell volume by the hematocrit method. Third edition. NCCLS publication H7-A3. Wayne, Pennsylvania: NCCLS, 2000.
- Linné JJ, Ringsrud KM. Clinical Laboratory Science, the Basics and Routine Techniques. Fourth edition. St. Louis, Missouri: Mosby, 1999: 279-95.
- http://www.beckman.com/products/instrument/
partchar/technology/coulterprinciple.asp- Thomas L. Hematocrit (HCT). In: Thomas L, ed. Clinical laboratory diagnostics, use and assessment of clinical laboratory results. Frankfurt/Main: TH-Books-Verl.-Ges., 1998: 479-82.
- Known interfering substances. In: ABL555 OM. Ed. 0212F. Brønshøj: Radiometer Medical A/S, 2002: Chapter 1, 1-5. Code no. 989-289.
- Stott RAW, Hortin GL, Wilhite TR et al. Analytical artifacts in hematocrit measurements by whole-blood chemistry analyzers. Clin Chem 1995; 41, 2: 306-11.
- User-defined corrections. In: BG, Oxi, Lyte and Metabolite Systems RM. Ed. M. Brønshøj: Radiometer Medical A/S, 1999: Chapter 1.8, 6-7. Code no. 985-787.
- Correlation. In: ABL77 OM. Ed. K. Brønshøj: Radiometer Medical A/S, 2002: Chapter 5, 23. Code no. 989-555.
- Krarup T, Walcker KS. Guideline to measurement on the ABL555 using the user-specified fluid mode. Radiometer publication Bulletin No. 6-1998. Brønshøj: Radiometer Medical A/S, 1998.
- O’Connor G, Molloy AM, Daly L. Deriving a useful packed cell volume estimate from haemoglobin analysis. J Clin Pathol 1994; 47: 78-79.
- Weatherall MS, Sherry KM. An evaluation of the Spuncrit™ infra-red analyzer for measurement of haematocrit. Clin Lab Haem 1997; 19: 183-86.
- Sorell-Rashi LA, Tomasic M. Evaluation of automated methods of measuring hemoglobin and hematocrit in horses. AJVR 1998; 59, 12: 1519-22.
- http://www.i-stat.com/products/ctisheets/714178-14d.pdf.
- Graitcer PL, Goldsby JB, Nichaman MZ. Hemoglobins and hematocrits: are they equally sensitive in detecting anemias? Am J Clin Nutr 1981; 34, 1: 61-64.
- Young PC, Hamill , Wasserman RC. Evaluation of the capillary microhematocrit as a screening test for anemia in pediatric office practice. PEDIATRICS 1986; 78, 2: 206-09.
- Celkan T, Gür E, Can G et al. Anemic or not? The Türkish Journal of Pediatrics 2003; 45: 329-34.
- Performance characteristics. In: ABL700 Series RM. Ed. H. Brønshøj: Radiometer Medical A/S, 2002: Chapter 5, 46-47. Code no. 989-312.
- Jacobs DS, Kasten BL Jr, Demott WR et al. Laboratory Test Handbook. Second ed. Hudson, Cleveland: Lexi-comp inc, 1990: 490-91.
- Lock R, Francke K, Nötzli B. The whole-blood sampling handbook. Brønshøj: Radiometer Medical A/S, 2000.
References
- Soldin SJ, Rifai N, Hicks JMB. Biochemical Basis of Pediatric Disease. Second ed. Washington DC: AACC Press, 1995: 5.
- Kokholm G. Simultaneous measurements of blood pH, pCO2, pO2 and concentrations of hemoglobin and its derivatives – a multicenter study. Radiometer publication AS107. Copenhagen: Radiometer Medical A/S, 1991.
- http://www.nlm.nih.gov/medlineplus/encyclopedia.html Medline Plus encyclopedia
- Bull BS, Koepke JA, Simson E et al. Procedure for determining packed cell volume by the hematocrit method. Third edition. NCCLS publication H7-A3. Wayne, Pennsylvania: NCCLS, 2000.
- Linné JJ, Ringsrud KM. Clinical Laboratory Science, the Basics and Routine Techniques. Fourth edition. St. Louis, Missouri: Mosby, 1999: 279-95.
- http://www.beckman.com/products/instrument/
partchar/technology/coulterprinciple.asp- Thomas L. Hematocrit (HCT). In: Thomas L, ed. Clinical laboratory diagnostics, use and assessment of clinical laboratory results. Frankfurt/Main: TH-Books-Verl.-Ges., 1998: 479-82.
- Known interfering substances. In: ABL555 OM. Ed. 0212F. Brønshøj: Radiometer Medical A/S, 2002: Chapter 1, 1-5. Code no. 989-289.
- Stott RAW, Hortin GL, Wilhite TR et al. Analytical artifacts in hematocrit measurements by whole-blood chemistry analyzers. Clin Chem 1995; 41, 2: 306-11.
- User-defined corrections. In: BG, Oxi, Lyte and Metabolite Systems RM. Ed. M. Brønshøj: Radiometer Medical A/S, 1999: Chapter 1.8, 6-7. Code no. 985-787.
- Correlation. In: ABL77 OM. Ed. K. Brønshøj: Radiometer Medical A/S, 2002: Chapter 5, 23. Code no. 989-555.
- Krarup T, Walcker KS. Guideline to measurement on the ABL555 using the user-specified fluid mode. Radiometer publication Bulletin No. 6-1998. Brønshøj: Radiometer Medical A/S, 1998.
- O’Connor G, Molloy AM, Daly L. Deriving a useful packed cell volume estimate from haemoglobin analysis. J Clin Pathol 1994; 47: 78-79.
- Weatherall MS, Sherry KM. An evaluation of the Spuncrit™ infra-red analyzer for measurement of haematocrit. Clin Lab Haem 1997; 19: 183-86.
- Sorell-Rashi LA, Tomasic M. Evaluation of automated methods of measuring hemoglobin and hematocrit in horses. AJVR 1998; 59, 12: 1519-22.
- http://www.i-stat.com/products/ctisheets/714178-14d.pdf.
- Graitcer PL, Goldsby JB, Nichaman MZ. Hemoglobins and hematocrits: are they equally sensitive in detecting anemias? Am J Clin Nutr 1981; 34, 1: 61-64.
- Young PC, Hamill , Wasserman RC. Evaluation of the capillary microhematocrit as a screening test for anemia in pediatric office practice. PEDIATRICS 1986; 78, 2: 206-09.
- Celkan T, Gür E, Can G et al. Anemic or not? The Türkish Journal of Pediatrics 2003; 45: 329-34.
- Performance characteristics. In: ABL700 Series RM. Ed. H. Brønshøj: Radiometer Medical A/S, 2002: Chapter 5, 46-47. Code no. 989-312.
- Jacobs DS, Kasten BL Jr, Demott WR et al. Laboratory Test Handbook. Second ed. Hudson, Cleveland: Lexi-comp inc, 1990: 490-91.
- Lock R, Francke K, Nötzli B. The whole-blood sampling handbook. Brønshøj: Radiometer Medical A/S, 2000.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








