Printed from acutecaretesting.org
July 2005
Hemoglobin and its measurement
Normal cell function depends on a continuous supply of oxygen. As oxygen is consumed during cell metabolism, carbon dioxide is produced.
A principle function of blood is the delivery of oxygen (O2), present in inspired air, from the lungs to every cell in the body and delivery of carbon dioxide (CO2) from cells to the lungs, for elimination from the body in expired air.
These vital gas transport functions are dependent on the protein hemoglobin contained in erythrocytes (red blood cells). Each of the 5 × 1010 erythrocytes normally present in 1 mL of blood contains around 280 million hemoglobin molecules.
1. HEMOGLOBIN STRUCTURE AND FUNCTION
The hemoglobin (Hb) molecule is roughly spherical and comprises two pairs of dissimilar subunits (FIGURE 1).
Each of the subunits is a folded polypeptide chain (the globin portion) with a heme group (derived from porphyrin) attached.
At the center of each heme group is a single atom of iron in the ferrous (Fe2+) state. Thus heme is a metallo-porphyrin, incidentally responsible for the red color of blood.
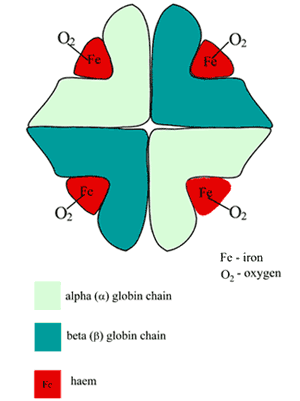
FIGURE 1: Schematic of oxygenated hemoglobin (HbA) structure
The oxygen-binding site of Hb is the heme pocket present in each of the four polypeptide chains; a single atom of oxygen forms a reversible bond with the ferrous iron at each of these sites, so a molecule of Hb binds four oxygen molecules; the product is oxyhemoglobin (O2Hb).
The oxygen delivery function of Hb, that is its ability to "pick up" oxygen at the lungs and "release" it to tissue cells is made possible by minute conformational changes in quaternary structure that occur in the hemoglobin molecule and which alter the affinity of the heme pocket for oxygen. Hb has two quaternary structural states: the deoxy state (low oxygen affinity) and the oxy state (high oxygen affinity).
A range of environmental factors determine the quaternary state of Hb and therefore its relative oxygen affinity. The microenvironment in the lungs favors the oxy-quaternary state, and thus Hb has high affinity for oxygen here.
By contrast, the microenvironment of the tissues induces the conformational change in Hb structure that reduces its affinity for oxygen, thus allowing oxygen to be released to tissue cells.
1.1. HEMOGLOBIN AND CARBON DIOXIDE ELIMINATION
A small amount (up to 20 %) of CO2 is transported from the tissues to the lungs loosely bound to the N-terminal amino acid of the four globin polypeptide units of hemoglobin; the product of this combination is carbaminohemoglobin. However, most CO2 is transported as bicarbonate in blood plasma.
The erythrocyte conversion of CO2 to bicarbonate, necessary for this mode of CO2 transport, results in the production of hydrogen ions (H+). These hydrogen ions are buffered by deoxygenated hemoglobin.
The role of hemoglobin in transport of oxygen and carbon dioxide is summarized in FIGURES 2a and 2b.
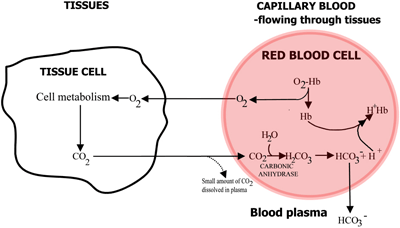
FIGURE 2a: TISSUES O2 diffuses from blood to tissues, CO2 diffuses from tissues to blood
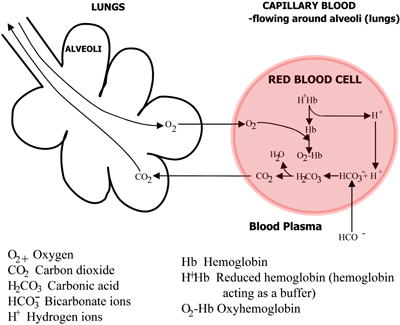
FIGURE 2b: LUNGS CO2 diffuses from blood to lungs, O2 diffuses from lungs to blood
In capillary blood flowing through the tissues oxygen is released from hemoglobin and passes into tissue cells. Carbon dioxide diffuses out of tissue cells into erythrocytes, where the red-cell enzyme carbonic anhydrase enables its reaction with water to form carbonic acid.
The carbonic acid dissociates to bicarbonate (which passes into the blood plasma) and hydrogen ions, which combine with the now deoxygenated hemoglobin. The blood flows to the lungs, and in the capillaries of the lung alveoli the above pathways are reversed. Bicarbonate enters erythrocytes and here combine with hydrogen ions, released from hemoglobin, to form carbonic acid.
This dissociates to carbon dioxide and water. The carbon dioxide diffuses from the blood into the alveoli of the lungs and is eliminated in expired air. Meanwhile, oxygen diffuses from the alveoli to capillary blood and combines with hemoglobin.
1.2. HEMOGLOBIN THAT CANNOT BIND OXYGEN
Although normally present in only trace amounts, there are three species of hemoglobin: methemoglobin (MetHb or Hi), sulfhemoglobin (SHb) and carboxyhemoglobin (COHb) which cannot bind oxygen.
They are thus functionally deficient, and increased amounts of any of these hemoglobin species, usually the result of exposure to specific drugs or environmental toxins, can seriously compromise oxygen delivery.
A comprehensive account of hemoglobin structure and function is provided in reference [1].
ctHb, the total hemoglobin concentration is typically defined as the sum of oxygenated hemoglobin, deoxygenated hemoglobin, carboxyhemoglobin and methemoglobin.
2. CLINICAL UTILITY OF ctHb MEASUREMENT
2.1. ANEMIA
The principle reason for measuring ctHb is detection of anemia and assessment of its severity.
Anemia can be defined as a reduction in the oxygen-carrying capacity of blood due to a reduction in erythrocyte numbers and/or a reduction in ctHb, so that anemia is established if ctHb is below the lower limit of reference (normal) range [2] (TABLE I). The lower the ctHb, the more severe is the anemia.
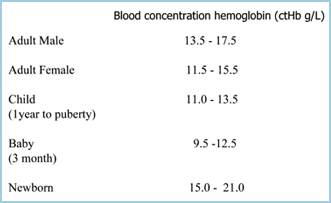
TABLE I: ctHb reference ranges (Ref 2)
Anemia is not a disease entity, rather a consequence or sign of disease. The reason why ctHb is such a frequently requested blood test is that anemia is a feature of a range of pathologies, many of which are relatively common (Table II).
Common symptoms, most of which are non-specific, include: pallor, tiredness and lethargy, shortness of breath – particularly on exertion, dizziness and fainting, headaches, constipation and increased pulse rate, palpitations, tachycardia.
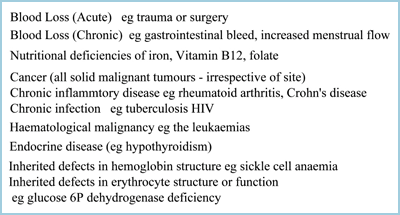
TABLE II: Some of the clinical conditions associated with anemia
The absence of these symptoms does not preclude anemia; many mildly anemic individuals remain asymptomatic, particularly if anemia has developed slowly.
2.2. POLYCYTHEMIA
Whilst anemia is characterized by reduced ctHb, a raised ctHb indicates polycythemia. Polycythemia arises as a response to any physiological or pathological condition in which blood contains less oxygen than normal (hypoxemia).
The body’s response to hypoxemia includes increased erythrocyte production to increase oxygen delivery, and as a consequence ctHb is raised. This so-called secondary polycythemia is part of the physiological adaptation to high altitude and may be a feature of chronic lung disease.
Primary polycythemia is a much less common malignancy of the bone marrow called polycythemia vera, which is characterized by uncontrolled production of all blood cells, including erythrocytes. Polycythemia, whether secondary or primary, is generally much less common than anemia.
3. MEASUREMENT OF ctHb
3.1. HISTORICAL PERSPECTIVE
The first clinical test of Hb measurement devised more than a century ago [3] involved adding drops of distilled water to a measured volume of blood until its color matched that of an artificial colored standard.
A later modification [4] involved first saturating blood with coal gas (carbon monoxide) to convert hemoglobin to the more stable carboxyhemoglobin. Modern hemoglobinometry dates from the 1950s, following development of spectrophotometry and the hemiglobincyanide (cynamethemoglobin) method.
Adaptation of this method and others for use in automated hematology analyzers followed. Over the past two decades advances have focused on development of methods which allow point-of-care testing (POCT) of hemoglobin.
This section deals first with consideration of some of the methods currently used in the laboratory and then with those POCT methods used outside the laboratory.
3.2. HEMIGLOBINCYANIDE - A SPECTROPHOTOMETRIC METHOD
Nearly 40 years after it was first adopted as the reference method for measuring hemoglobin by the International Committee for Standardization in Hematology (ICSH) [5], the hemiglobincyanide (HiCN) test remains the recommended method of the ICSH [6] against which all new ctHb methods are judged and standardized.
The detailed consideration that follows reflects its continued significance both as a reference and routine laboratory method.
3.2.1. Test principle
Blood is diluted in a solution containing potassium ferricyanide and potassium cyanide. Potassium ferricyanide oxidizes the iron in heme to the ferric state to form methemoglobin, which is converted to hemiglobincyanide (HiCN) by potassium cyanide.
HiCN is a stable colored product, which in solution has an absorbance maximum at 540 nm and strictly obeys Beer-Lambert’s law. Absorbance of the diluted sample at 540 nm is compared with absorbance at the same wavelength of a standard HiCN solution whose equivalent hemoglobin concentration is known.
Most hemoglobin derivatives (oxyhemoglobin, methemoglobin and carboxyhemoglobin, but not sulfhemoglobin) are converted to HiCN and therefore measured by this method.
3.2.1.1. Reagent diluent (modified Drabkin solution) [7]
| Potassium ferricyanide (K3Fe(CN)6) | 200 mg |
| Potassium cyanide (KCN) | 50 mg |
| Dihydrogen potassium phosphate (KH2 PO4) | 140 mg |
| Non-ionic detergent (e.g. Triton X-100) | 1 mL |
| Above diluted to 1000 mL in distilled water |
3.2.1.2. Manual method
25 µl of blood is added to 5.0 mL reagent, mixed and left for 3 minutes. Absorbance is read at 540 nm against a reagent blank. The absorbance of HiCN standard is measured in the same way.
3.2.1.3. ICSH HiCN standard
The major advantage of this method is that there is a standard HiCN solution manufactured and assigned a concentration value according to very precise criteria laid down and reviewed periodically by the International Council for Standardization in Hematology (ICSH) [6].
This international standard solution is the primary calibrant for the commercial standard solutions used in clinical laboratories around the world. Thus all those using HiCN standardization are effectively using the same standard, whose value has been scrupulously validated.
3.2.1.4. Interference
Turbidity due to proteins, lipids and cellular matter is a potential problem with spectrophotometric estimation of any blood constituent, including hemoglobin.
The large dilution (1:251) of sample largely eliminates the problem, but falsely raised ctHb results can occur in patients whose plasma protein concentration is particularly high [8,9,10].
Heavily lipemic samples and those containing very high numbers of white cells (leucocytes) can also artefactually raise ctHb by a similar mechanism [11].
3.2.1.5. Advantages of HiCN
- International standard – accurate
- Easily adapted to automated hematology analyzers; thus reproducible (low SD and CV – within batch CV typically < 0.5 %)
- Well established and thoroughly investigated – ICSH recommended
- Inexpensive reagent
3.2.1.6. Disadvantages of HiCN
- Manual method requires accurate pipetting and spectrophotometer
- Reagent (cyanide) hazardous
- The above limit its use outside the laboratory
- Subject to interference from raised lipids, plasma proteins and leucocyte numbers
- Does not distinguish those hemoglobin derivatives which have no oxygen-carrying capacity (MetHb, COHb, SHb). Thus may overestimate the oxygen-carrying capacity of blood if these are present in abnormal (more than trace) amounts.
3.3. ALTERNATIVE (CYANIDE-FREE) LABORATORY METHODS
3.3.1. Sodium Lauryl Sulphate method
Sodium Lauryl Sulphate (SLS) is a surfactant which both lyses erythrocytes and rapidly forms a complex with the released hemoglobin. The product SLS-MetHb is stable for a few hours and has a characteristic spectrum with maximum absorbance at 539 nm [12].
The complex obeys Beer-Lambert’s law so there is precise linear correlation between Hb concentration and absorbance of SLS-MetHb.
The method simply involves mixing 25 µL of blood with 5.0 mL of a 2.08-mmol/L solution of SLS (buffered to pH of 7.2), and reading absorbance at 539 nm. The results of ctHb by the SLS-Hb method have been shown to correlate very closely (r = 0.998) with the reference HiCN method [13].
The method has been adapted for automated hematology analyzers and is as reliable in terms of both accuracy and precision as automated HiCN methods [13,14,15]. A major advantage is that the reagent is non-toxic. It is also less prone to interference by lipemia and increased concentration of leukocytes [13].
The long-term instability of SDS-MetHb precludes its use as a standard so the method must be calibrated with blood whose ctHb has been determined using the reference HiCN method.
3.3.2. Azide-methemoglobin method
This method is based on conversion of hemoglobin to a stable colored product azide-methemoglobin which has an almost identical absorbance spectrum to that of HiCN [16].
The reagent used in this method is very similar to that used in the HiCN reference method with substitution of sodium azide for the more toxic potassium cyanide. As in the HiCN method, hemoglobin is converted to methemoglobin by potassium ferricyanide; azide then forms a complex with methemoglobin.
ctHb results by this method are comparable to results obtained by reference HiCN method; this is an acceptable alternative manual method. The explosive potential of sodium azide, however, prevents its use on automated hematology analyzers [17]. The azide-MetHb reaction has been adapted for POCT hemoglobinometers.
3.4. MEASURING ctHb OUTSIDE THE LABORATORY
The POCT methods considered here are:
- Portable hemoglobinometers
- CO-oximetry – a method utilized in POCT blood gas analyzers
- WHO color scale
3.4.1. Portable hemoglobinometers
Portable hemoglobinometers like the HemoCue-B allow accurate determination of hemoglobin at the bedside. They are essentially photometers which allow measurement of color intensity of solutions.
The disposable microcuvette in which these measurements are made also acts as reaction vessel. The reagents necessary for both release of Hb from erythrocytes and conversion of Hb to a stable colored product are present in dried form on the walls of the cuvette.
All that is required is introduction of a small sample (typically 10 µL) of capillary, venous or arterial blood to the microcuvette and insertion of the microcuvette into the instrument.
The instrument is factory precalibrated using HiCN standard, and absorbance of the test solution is automatically converted to ctHb. Result is displayed in less than a minute.
3.4.1.1. Advantages of modern hemoglobinometers include
- Portability
- Battery or mains operated, can be used anywhere
- Small sample volume (10 µL) obtained by finger prick
- Fast (result in 60 seconds)
- Ease of use – no pipetting
- Minimal training required by non-laboratory staff
- Standardized against HiCN – results comparable to those obtained in laboratory
- Correction for turbidity. In this respect portable hemoglobinometers superior to most ctHb methods [18].
This technology has been extensively evaluated in a range of settings and most studies [18-24] have confirmed acceptable accuracy and precision when compared with laboratory methods.
3.4.1.2. Disadvantages
Some studies [23,25], however, have raised concern that in the hands of non-laboratory staff results may be less satisfactory. Despite the simplicity of operation these instruments are not immune from operator error, and effective training is essential.
There is evidence to suggest that results derived from capillary (finger prick) samples are less precise than those derived from well-mixed capillary or venous samples collected into EDTA bottles [25].
3.4.2. CO-oximetry
A CO-oximeter is a specialized spectrophotometer, the name reflecting the original application, which was to measure COHb and MetHb.
Many modern blood gas analyzers have an incorporated CO-oximeter, allowing the simultaneous estimation of ctHb during blood gas analysis.
The measurement of ctHb by CO-oximetry is based on the fact that hemoglobin and all its derivatives are colored proteins which absorb light at specific wavelengths and thus have a characteristic absorbance spectrum (FIGURE 3).
Beer-Lambert’s law dictates that absorbance of a single compound is proportional to the concentration of that compound. If the spectral characteristic of each absorbing substance in a solution is known, absorbance readings of the solution at multiple wavelengths can be used to calculate the concentration of each absorbing substance.
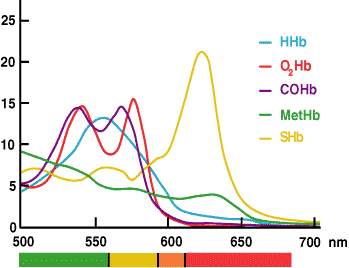
FIGURE 3.
In the CO-oximeter absorbance measurements of a hemolyzed blood sample at multiple wavelengths across the range that hemoglobin species absorb light (520-620 nm) are used by the installed software to calculate the concentration of each of the hemoglobin derivatives (HHb, O2Hb, MetHb and COHb). ctHb is the calculated sum of these derivatives.
All that is required from the operator is injection of a well-mixed arterial blood sample into the blood gas analyzer/CO-oximeter.
The sample, or a portion of it, is automatically pumped to the measuring cuvette of the CO-oximeter, where – by either chemical or physical action – erythrocytes are lysed to release hemoglobin, which is spectroscopically scanned as described above.
Results are displayed along with blood gas results within a minute or two.
Several studies [26,27,28] have confirmed that ctHb results obtained by CO-oximetry are not clinically significantly different from those derived from reference laboratory methods. CO-oximetry provides an acceptable means of urgent estimation of ctHb in a critical care setting.
3.4.2.1. The particular advantages of ctHb by CO-oximetry include
- Speed of analysis
- Ease of analysis
- Small sample volume
- No capital or consumable cost beyond that required for blood gas analysis
- Additional parameters (MetHb, COHb, O2Hb) measured
- Not affected by high white-cell count [29]
3.4.3. WHO hemoglobin color scale (HCS)
Developed for the World Health Organization (WHO), this low-technology test has limited application in developed countries but has huge significance for the economically deprived countries of the developing world, where anemia is most prevalent.
In areas where there are no laboratory facilities and insufficient resources to fund more sophisticated POCT hemoglobinometers, it is virtually the only means of determining ctHb.
The HCS test is based on the simple principle that the color of blood is a function of ctHb. A drop of blood is absorbed onto paper and its color compared with a chart of six shades of red, each shade representing an equivalent ctHb: the lightest 40 g/L and the darkest 140 g/L. Although in principle very simple, considerable research and technology was used in development to ensure maximum possible accuracy and precision [30].
For example, extensive trials of different papers informed the final choice of paper for the test strip matrix, and spectrophotometric analysis of blood and dye mixtures were employed to arrive at the closest possible match between chart color and the color of blood at each reference ctHb.
3.4.3.1. Advantages of the HCS test
- Is easy to use – requires only 30 minutes training
- Requires no equipment or power
- Is fast – result within 1 minute
- Requires only a finger prick (capillary) sample
- Is very cheap (around USD 0.12 per test)
3.4.3.2. Disadvantages of the HCS test
Reliable results depend on strict adherence to test instructions [31].
Common errors include:
- Inadequate or excessive blood on the test strip
- Reading result too late (beyond 2 minutes) or too soon (less than 30 sceonds)
- Reading the result under poor lighting conditions
The HSC test clearly has inherent limitations [32]. At best it can determine that the ctHb of a patient sample lies within one of six concentration ranges: 30-50 g/L, 50-70 g/L, 70-90 g/L, 90-110 g/L, 110-130 g/L or 130-150 g/L. Still this is theoretically sufficient to identify all but the most mildly anemic patients and give an indication of severity.
An early study [30] demonstrated the test's ability to identify anemia (defined as ctHb < 120 g/L) in 1213 samples with a sensitivity of 91 % and specificity of 86 %. Subsequent trials [31,33] have confirmed that it is an acceptable clinical tool to screen for anemia in the absence of more sophisticated technology and is significantly more sensitive and more specific than clinical examination.
4. SUMMARY
ctHb is one of two parameters routinely used to assess the oxygen-carrying capacity of blood and thereby establish a diagnosis of anemia and polycythemia.
The alternative test, called the hematocrit (Hct) or Packed Cell Volume (PCV), was the subject of a previous companion article, where the relationship between ctHb and Hct was discussed [34]. The focus of this article has been measurement of ctHb.
Numerous methods have been devised, the majority based on measuring the color of hemoglobin or a derivative of hemoglobin. For this short review it has inevitably been necessary to be selective. The methods chosen for discussion are among the most commonly used today.
In making the selection an attempt has been made to convey the range of technologies that are currently employed and how these are applied to satisfy the clinical demand for ctHb in settings that range from impoverished areas of the developing world, where medical care barely has a foothold, to the high-tech world of the modern intensive care unit.
References+ View more
- Ranney HM, Sharma V. Structure and Function of Hemoglobin. In Beutler E Lichtman M et al (eds). Willimas’s Hematology. 6th edition. McGraw Hill, 2000: 345-53.
- Hoffbrand AV, Pettit JE. Essential Haematology. 3rd edition. Oxford: Blackwell, 1993: 24-25.
- Gowers WR An apparatus for the clinical estimation of haemoglobin. Trans. Clin. Soc. Lond., 1879; 12: 64-67.
- Haldane J. The colorimetric determination of haemoglobin. J Physiol 1901; 26: 497-04.
- ICSH. Recommendations for haemoglobinometry in human blood. Br J Haematol. 1967; 13 (suppl:71-6).
- ICSH Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobincyanide standard. 4th edition. J Clin Path 1996; 49: 271-74.
- van Kampen EJ, Zijlstra WJ. Standardization of hemoglobinometry II The hemiglobincyanide method. Clin Chim Acta 1961; 6: 538-44
- Roberts W, Fontenot J D, Lehman C M. Overestimation of hemoglobin in a patient with an IgA-κ monoclonal Gammopathy. Arch Pathol Lab Med 2000; 124: 616-18.
- Wallis JP, Ford JM. Incorrect hemoglobin estimation on the Coulter S in some patients with IgM paraproteins. Clin Lab Haematol 1987; 9: 95-96
- Malin MJ, Sclafani LD, Wyatt JL. Evaluation of 24-second cyanide-containing and cyanide-free methods for whole blood hemoglobin on the Technicon H*1TM analyzer with normal and abnormal blood samples. Am J Clin Pathol 1989; 33: 117-30
- Seigneurin D, Passe D. Interference of hyperleukocytosis on Coulter Model S Blood counts; methods for correction. Biomedicine & Pharmacotherapy 1983; 37: 401-04
- Oshiro I, Takenaka T, Maeda J. New method for hemoglobin
determination by using sodium lauryl sulfate (SLS). Clin Biochem 1982; 15: 83-88- Lewis SM, Garvey B, Manning R, Sharp SA, Wardle J. Lauryl sulphate haemoglobin: a non-hazardous substitute for HiCN in haemoglobinometry. Clin Lab Haematol 1991; 13: 279-90
- Karsan A, Maclaren I, Conn D, Wadsworth L. An evaluation of hemoglobin determination using sodium lauryl sulfate. Am J Clin Path 1993;100: 123-26
- Harris N, Devoto G, Pappas J et al. Performance evaluation of the ADVIA 2120 hematology analyzer: an international multicenter clinical trial. Lab Hematol 2005; 11: 62-70
- Vanzetti G. An azide-methemoglobin method for hemoglobin determination in blood. J Lab & Clin Med 1966; 67: 116-26
- van Assendelft OW, Horton BR, Parvin RM. Calibration and control in hemoglobinometry. Clin Lab Haematol 1990; 12 (suppl 1): 331-42
- von Schenk H, Falkensson M, Lundberg B. Evaluation of “HemoCue”, a new device for determining hemoglobin. Clin Chem 1986 32: 526-29
- Rosenbilt J, Abreu C, Szterling L et al. Evaluation of three methods for hemoglobin measurement in a blood donor setting. Sao Paulo Med J 1999; 117: 108-12
- Sharp S, Lewis SM, Williams SK. Evaluation of the HemoCue B-haemoglobin photometer. Medical Devices Agency evaluation report MDA/95/21 1995. London HMSO
- Lardi AM, Hirst C, Mortimer AJ, MCollum CN. Evaluation of HemoCue for measuring intra-operative haemoglobin concentrations: a comparison with Coulter Max-M. Anaesthesia 1998;53: 349-52
- Munoz M, Romero A, Gomez JF, Manteca A, Naveira E, Ramirez G. Utility of point-of-care measurement in the HemoCue-B haemoglobin for the initial diagnosis of anaemia. Clin Lab Haematol 2005; 27: 99-104
- Neville RG. Evaluation of portable haemoglobinometer in general practice. Br Med J 1987; 294: 1263-65
- Medina Lara A, Mundy C, Kandulu J, Chisuwo L, Bates I. Evaluation and costs of different haemoglobin methods for use in district hospitals in Malawi. J Clin Pathol 2005; 58: 56-60
- Conway A, Hinchliffe RF, Earland J, Anderson LM. Measurement of haemoglobin using single drops of skin puncture blood: is precision acceptable? J Clin Pathol 1998; 51: 248-50
- Dennis RC, Valeri CR. Measuring percent oxygen saturation of hemoglobin, percent carboxyhemoglobin and methemoglobin, and concentrations of total hemoglobin and oxygen in blood of man, dog, and baboon. Clin Chem 1980; 26: 1304-08
- Ray J, Post J, Hamielec C. Use of rapid arterial blood gas analyser to estimate hemoglobin concentration among critically ill adults. Critical Care 2001; 672-75
- Gehring H, Hornberger C, Dibbelt L et al. Accuracy of point-of care testing (POCT) for determining hemoglobin concentrations. Acta Anaesthesiol Scand 2002 46: 980-86
- Scharnhorst V, van der Laar PJ, Vader HL. Hemoglobin in samples with leukocytosos can be measured on the ABL 700 Series blood gas analyzers. Clin Chem 2003; 49: 2107-08.
- Lewis SM, Stott GJ, Wynn KJ. An inexpensive and reliable new haemoglobin colour scale for assessing anaemia. J Clin Path 1998; 51: 21-24
- Paddle J. Evaluation of the Haemoglobin Colour Scale and comparison with HemoCue haemoglobin assay. Bulletin of the World Health Organisation. 2002; 80: 813-16
- Ingram CF, Lewis SM. Clinical use of WHO haemoglobin colour scale: validation and critique. J Clin Pathol 2000; 53: 933-37
- Timan IS, Tatsumi N, Aulia D et al. Comparison of haemoglobinometry by WHO Haemoglobin Colour Scale and copper sulphate against haemiglobincyanide reference method. Clin Lab Haematol 2004 26: 253-58.
- Wennecke G. Hematocrit – a review of different analytical methods. www.acutecaretesting.org 2004
References
- Ranney HM, Sharma V. Structure and Function of Hemoglobin. In Beutler E Lichtman M et al (eds). Willimas’s Hematology. 6th edition. McGraw Hill, 2000: 345-53.
- Hoffbrand AV, Pettit JE. Essential Haematology. 3rd edition. Oxford: Blackwell, 1993: 24-25.
- Gowers WR An apparatus for the clinical estimation of haemoglobin. Trans. Clin. Soc. Lond., 1879; 12: 64-67.
- Haldane J. The colorimetric determination of haemoglobin. J Physiol 1901; 26: 497-04.
- ICSH. Recommendations for haemoglobinometry in human blood. Br J Haematol. 1967; 13 (suppl:71-6).
- ICSH Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobincyanide standard. 4th edition. J Clin Path 1996; 49: 271-74.
- van Kampen EJ, Zijlstra WJ. Standardization of hemoglobinometry II The hemiglobincyanide method. Clin Chim Acta 1961; 6: 538-44
- Roberts W, Fontenot J D, Lehman C M. Overestimation of hemoglobin in a patient with an IgA-κ monoclonal Gammopathy. Arch Pathol Lab Med 2000; 124: 616-18.
- Wallis JP, Ford JM. Incorrect hemoglobin estimation on the Coulter S in some patients with IgM paraproteins. Clin Lab Haematol 1987; 9: 95-96
- Malin MJ, Sclafani LD, Wyatt JL. Evaluation of 24-second cyanide-containing and cyanide-free methods for whole blood hemoglobin on the Technicon H*1TM analyzer with normal and abnormal blood samples. Am J Clin Pathol 1989; 33: 117-30
- Seigneurin D, Passe D. Interference of hyperleukocytosis on Coulter Model S Blood counts; methods for correction. Biomedicine & Pharmacotherapy 1983; 37: 401-04
- Oshiro I, Takenaka T, Maeda J. New method for hemoglobin
determination by using sodium lauryl sulfate (SLS). Clin Biochem 1982; 15: 83-88- Lewis SM, Garvey B, Manning R, Sharp SA, Wardle J. Lauryl sulphate haemoglobin: a non-hazardous substitute for HiCN in haemoglobinometry. Clin Lab Haematol 1991; 13: 279-90
- Karsan A, Maclaren I, Conn D, Wadsworth L. An evaluation of hemoglobin determination using sodium lauryl sulfate. Am J Clin Path 1993;100: 123-26
- Harris N, Devoto G, Pappas J et al. Performance evaluation of the ADVIA 2120 hematology analyzer: an international multicenter clinical trial. Lab Hematol 2005; 11: 62-70
- Vanzetti G. An azide-methemoglobin method for hemoglobin determination in blood. J Lab & Clin Med 1966; 67: 116-26
- van Assendelft OW, Horton BR, Parvin RM. Calibration and control in hemoglobinometry. Clin Lab Haematol 1990; 12 (suppl 1): 331-42
- von Schenk H, Falkensson M, Lundberg B. Evaluation of “HemoCue”, a new device for determining hemoglobin. Clin Chem 1986 32: 526-29
- Rosenbilt J, Abreu C, Szterling L et al. Evaluation of three methods for hemoglobin measurement in a blood donor setting. Sao Paulo Med J 1999; 117: 108-12
- Sharp S, Lewis SM, Williams SK. Evaluation of the HemoCue B-haemoglobin photometer. Medical Devices Agency evaluation report MDA/95/21 1995. London HMSO
- Lardi AM, Hirst C, Mortimer AJ, MCollum CN. Evaluation of HemoCue for measuring intra-operative haemoglobin concentrations: a comparison with Coulter Max-M. Anaesthesia 1998;53: 349-52
- Munoz M, Romero A, Gomez JF, Manteca A, Naveira E, Ramirez G. Utility of point-of-care measurement in the HemoCue-B haemoglobin for the initial diagnosis of anaemia. Clin Lab Haematol 2005; 27: 99-104
- Neville RG. Evaluation of portable haemoglobinometer in general practice. Br Med J 1987; 294: 1263-65
- Medina Lara A, Mundy C, Kandulu J, Chisuwo L, Bates I. Evaluation and costs of different haemoglobin methods for use in district hospitals in Malawi. J Clin Pathol 2005; 58: 56-60
- Conway A, Hinchliffe RF, Earland J, Anderson LM. Measurement of haemoglobin using single drops of skin puncture blood: is precision acceptable? J Clin Pathol 1998; 51: 248-50
- Dennis RC, Valeri CR. Measuring percent oxygen saturation of hemoglobin, percent carboxyhemoglobin and methemoglobin, and concentrations of total hemoglobin and oxygen in blood of man, dog, and baboon. Clin Chem 1980; 26: 1304-08
- Ray J, Post J, Hamielec C. Use of rapid arterial blood gas analyser to estimate hemoglobin concentration among critically ill adults. Critical Care 2001; 672-75
- Gehring H, Hornberger C, Dibbelt L et al. Accuracy of point-of care testing (POCT) for determining hemoglobin concentrations. Acta Anaesthesiol Scand 2002 46: 980-86
- Scharnhorst V, van der Laar PJ, Vader HL. Hemoglobin in samples with leukocytosos can be measured on the ABL 700 Series blood gas analyzers. Clin Chem 2003; 49: 2107-08.
- Lewis SM, Stott GJ, Wynn KJ. An inexpensive and reliable new haemoglobin colour scale for assessing anaemia. J Clin Path 1998; 51: 21-24
- Paddle J. Evaluation of the Haemoglobin Colour Scale and comparison with HemoCue haemoglobin assay. Bulletin of the World Health Organisation. 2002; 80: 813-16
- Ingram CF, Lewis SM. Clinical use of WHO haemoglobin colour scale: validation and critique. J Clin Pathol 2000; 53: 933-37
- Timan IS, Tatsumi N, Aulia D et al. Comparison of haemoglobinometry by WHO Haemoglobin Colour Scale and copper sulphate against haemiglobincyanide reference method. Clin Lab Haematol 2004 26: 253-58.
- Wennecke G. Hematocrit – a review of different analytical methods. www.acutecaretesting.org 2004
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars








