Printed from acutecaretesting.org
March 2021
Improving clinical care and reducing health-care cost: the strength of using PCT to guide diagnosis and treatment
Content
- Introduction
- Diagnosing and treating sepsis
- Can PCT testing improve health care?
- Can PCT testing reduce health-care cost?
- Can PCT testing improve health care at reduced cost?
Introduction
Procalcitonin (PCT) is a 116 amino acid prohormone of calcitonin and katacalcin, both involved with bone mineralization. In healthy individuals, plasma levels of procalcitonin (PCT) are usually non-detectable, e.g. at levels below 0.05 ng/mL, and is produced and processed in the thyroid gland. In response to inflammation, especially when caused by bacterial infection, PCT is also produced and released into the blood by many other organs, causing PCT levels to increase dramatically. Within 4-6 hours after the beginning of infection, PCT levels often exceed 0.25 ng/mL, reaching maximum levels after about 24-48 hours with concentrations up to 100 ng/mL or even higher, depending on the nature and course of the infection. PCT is considered a sensitive and rather specific marker for the presence of bacterial infection and sepsis in a wide range of patients [1]. Also, plasma levels of PCT correlate with severity of disease and can therefore offer valuable prognostic information for patients with infections, such as in sepsis and septic shock. [2, 3, 4]
Aside bacterial infection, such as e.g. sepsis, other triggers are reported to elevate levels of PCT and occasionally cause PCT levels around or even above the diagnostic cut-off. Situations where the PCT elevations may be due to a non-bacterial cause include:
- Newborns (first 2-3 days)
- Massive stress (severe trauma, surgery, cardiac shock, burns)
- Treatment cytokines stimulating agents
- Malaria and in some cases of fungal and viral infections
- Prolonged, severe cardiogenic shock or organ perfusion abnormalities
- Some forms of vasculitis and acute graft vs. host disease
- Paraneoplastic syndromes due to medullary thyroid and small cell lung cancer
- Significantly compromised renal function, especially ESRD/hemodialysis
Although the above inflammatory stimuli can cause elevated PCT levels and should always be taken into consideration when finding increased PCT results, their effect is mostly less significant than the effect caused by bacterial infection and sepsis.
Diagnosing and treating sepsis
According to the current definition (Sepsis-3), sepsis is essentially (newly acquired) organ dysfunction related to an ongoing infection [5]. While organ dysfunction is typically scored through a panel of biomarkers, e.g. SOFA, qSOFA, NEWS, or MEDS, a convenient and rapid assessment of sepsis is still a challenge for the current standard of care. Traditionally, bacterial infections such as present in sepsis will be diagnosed based on a positive culture from blood or other patients’ specimen. Unfortunately, this traditional procedure will require at least a few days before results can be shared with the treating physician. For obvious reasons and in accordance with current recommendations, antibiotic treatment (ABx) with empiric antibiotics should be initiated preferably within 1 hour after admission, while samples for cultures are to be collected prior to start of ABx. Reassessment of the effect of ABx should be performed regularly [6].
Because most infections have a viral etiology, ABx is often applied unwarranted and should be discontinued when bacterial infection can be excluded [7]. Because PCT levels can help in the early assessment whether a patient is burdened with bacterial sepsis [1], PCT testing can help to guide a more efficient use of antibiotics [9]. Consequently, PCT testing is currently under general consideration as an integrated diagnostic tool to monitor progression of disease and to guide ABx in patients burdened with bacterial sepsis [10]. Sepsis and septic shock are serious clinical conditions associated with high mortality rates and often lifelong levels of post-sepsis morbidity in survivors of sepsis [5]. In combination with a bedside clinical assessment (qSOFA), PCT has been shown to improve prognostic strength for mortality in patients with suspected sepsis [8]. Although the pathological escalation from a limited infection to a full-scale systemic sepsis is still poorly understood, mortality can be reduced when infections as present during sepsis are detected early and can be treated by non-delayed effective antibiotic therapy [11].
Irrespective of any biological and clinical considerations, the implementation of a new laboratory test in routine clinical diagnostics is only justifiable when such a new laboratory tool delivers at least in one of two categories:
- patient care improves
- patient care remains essentially the same, but at reduced health care cost
The “best case” scenario, a diagnostic tool improving patient care while reducing health-care cost, would justify a swift implementation in the standard-of-care. The following two chapters will discuss in which of these categories PCT testing should be classified. In case PCT would qualify for both above categories, a swift implementation in appropriate standard-of-care algorithms is justified and warranted.
Can PCT testing improve quality of care?
The earlier studies on PCT have focused primarily on the clinical diagnostic benefit of using this biomarker in patients with suspected bacterial infection in various clinical settings e.g. sepsis. By now, over 2000 peer reviewed articles have been published dedicated to aiding in the “rule-in” or “rule-out” of bacterial infection, including sepsis and septic shock. In more recent years, clinical research has focused more on developing PCT as a tool to indicate and optimize efficiency of antibiotic treatment in for example sepsis patients [9, 12].
Main areas in which PCT testing may provide diagnostic and clinical value:
- Indicate ABx should (not) be initiated in patients admitted to ED with suspected bacterial infection such as in sepsis [10, 13]
- Follow the effectiveness of ABx in stationary patients treated for bacterial infection such as in sepsis or septic shock [10, 12]
- Indicate if ABx could be stopped or should continue [10, 13]
Two important findings were reported in the early reports:
- PCT-guided ABx for sepsis patients allowed reduced use of antibiotics, e.g. fewer and shorter treatments
- Despite less use of antibiotics, PCT-guided ABx did not increase adverse outcomes
Several recent papers have developed and validated PCT cutoffs for patients with acute bacterial infections as found during sepsis and various other clinical settings [10, 12, 13]. Finally, consensus is emerging regarding the optimal PCT-levels for initiation or discontinuation of antibiotic therapy in the ED or ICU [10].
Many patients admitted to ED with suspected bacterial infections such as e.g. sepsis, suffer in fact from viral infections and hence, do not profit from ABx. Still, many of such patients receive ABx, which is associated with avoidable costs and avoidable drug associated adverse effects. [10-14]. Consequently, the potential clinical diagnostic and socioeconomic benefits of PCT testing in these patient groups are easy to understand.
Figure 1, 2, and 3. The three algorithms (Adapted from Schuetz et al. [10].
Patient with mild illness outside ICU
(Defined by setting specific scores, e.g. qSOFA, MEDS, NEWS)
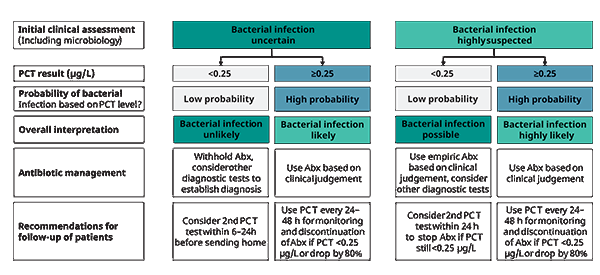
Figure 1. PCT use in patients with mild illness, i.e. indicative of mild symptoms of sepsis, outside the ICU
Patient with moderate illness outside ICU
(Defined by setting specific scores, e.g. qSOFA, MEDS, NEWS)
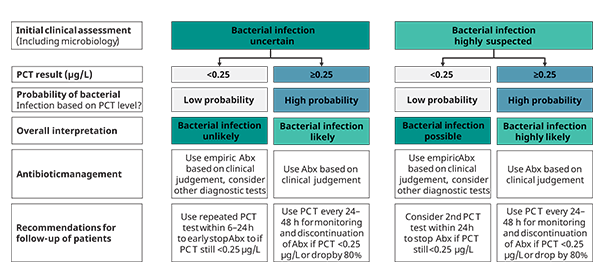
Figure 2. PCT use in patients with moderate illness, i.e. indicative of moderate symptoms of sepsis, outside the ICU
Patient with severe illness in ICU
(Defined by setting specific scores, e.g. qSOFA, SOFA, APACHE)
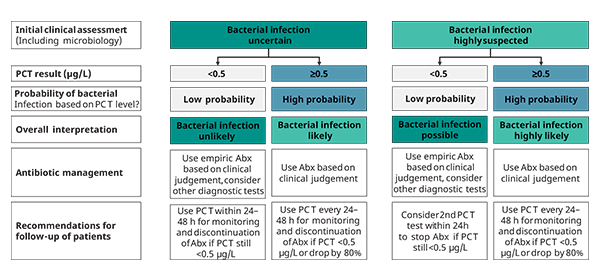
Figure 3. PCT use in patients with severe illness, i.e. indicative of severe symptoms of sepsis, in the ICU
*For figures 1-3: Caution in patients with immuno-suppression (including HIV), CF, pancreatitis, trauma, pregnancy, high volume transfusion, malaria; PCT-guided stewardship should not be applied to patients with chronic infections (e.g. abscess, osteomyelitis, endocarditis)
For figures 1-3: Permission obtained through Copyright Clearance Center
Recently, an expert group published recommendations for optimized antibiotic therapy for patients with symptoms indicative of sepsis based on PCT results, summarized in figures 1-3. The presented algorithms are adjusted for disease severity, for pre-test suspicion of infection, and for the location of the patient (in- or outside ICU). According to this expert’s consensus paper, PCT guided treatment has the potential to improve the diagnostic and therapeutic management of patients suspected having bacterial infection, such as present in sepsis [10]. Moreover, the proposed algorithms for optimal use of PCT results for patients with symptoms indicative of sepsis seem to provide additional clinical value through reduced mortality [15-18] and a reduced number of drug associated adverse effects, including less infections with multi-drug resistant bacteria, and a lower prevalence of infections with Clostridium difficile (C. diff), a problem especially seen in the USA [18-20]. Also, depending on the type or combination of antibiotic(s) applied, fewer side effects have been observed. [21].
Considering all the above, PCT qualifies as a biomarker supporting improved patient care, by helping to diagnose bacterial infections as observed during sepsis, providing prognostic value regarding the severity, and by reducing the number of antibiotic related side effects.
Finally, using PCT to guide antibiotic treatment for sepsis patients appears to save costs for the health-care systems in several clinical settings, especially in the ICU. We will discuss this in more detail in the next chapter.
Can PCT testing reduce health-care cost?
The cost efficiency of a new diagnostic algorithm using PCT to guide antibiotic treatment very much depends on the clinical setting and on the health-care system in question, as the health-care cost structure of various countries will differ significantly. Moreover, most models used to calculate the effects of a new algorithm on health-care costs consider a limited number of clinical and socio-economic variables. In other words, we should always appreciate and critically examine the limitations and potential bias of publications calculating the effect on health-care costs of any new diagnostic algorithm.
In recent years, several publications, especially from Europe und US, have focused on the socio-economic value of PCT testing as a guide to treat e.g. sepsis patients [22-29].
The reduced health-care costs were mostly driven by shorter length of hospital stay, a lower need of antibiotics, and fewer antibiotic -related side effects. Because the studies and models are based on different health-care systems and underlying costs, and often use a different set of health-care efficiency variables, absolute results of the various reports are difficult to compare directly. Nonetheless, almost every socio-economic study using PCT to guide treatment of e.g. sepsis patients, reports significant savings despite the incremental costs associated with the PCT testing.
In more detail, the main contributors to the health-care cost reduction described in these reports were:
- Shorter length-of-stay in hospital wards, especially of patients in ICU with sepsis
- Shorter time on antibiotics, less need of antibiotics, and lower number of patients on antibiotics
- Lower numbers of antibiotic-related side-effects, e.g. lower prevalence of nosocomial and multi-drug resistant infections
- Reduced days on ventilation
Recently, Mewes et al. [19] have published a comprehensive US-based model for calculating the cost-efficiency of PCT-guided treatment of e.g sepsis patients, based mainly on US clinical reports and US cost-of-care, including major contributors such as length of hospital stay, loss of productivity, prevalence and cost of antibiotic resistance, cost of adverse effects, e.g. C. diff infections, etc. Ultimately, the socio-economic effects of adding PCT to guide antibiotic treatment in this model was calculated. The authors have reported savings of approximately 11,000 dollars for sepsis patients. Considering the vast number of such patients every year, the potential savings for the US health-care system would be tremendous.
Voermans et al. [20] have validated the above model in a real clinical setting and compared their local health-care costs for the management of patients with sepsis in a period prior to the introduction of PCT-guided antibiotics treatment. They confirm the findings of Mewes et al. and have extrapolated potential savings of 23-50% in the US health-care setting by the implementation of PCT-guided antibiotics treatment in these patients.
Recently, also Kyriazopoulou et al [30] demonstrated the long-term benefit of PCT testing of sepsis patients by reducing the number of adverse effects, such as C. diff or other infections, by reducing 28-days mortality from 28.2% to 15.2%, as well as by reducing the overall cost of patient management, especially by reduced need for antibiotics and shorter duration of hospitalization.
Can PCT testing improve health care at reduced cost?
There is an increasing body of evidence that PCT used to guide treatment of sepsis patients can improve quality of care and will reduce health-care costs in the management of patients suspected to have sepsis in ED and ICU settings.
In conclusion, the clinical benefit and cost effectiveness of PCT testing, especially in guiding antibiotic therapy in patients with suspected sepsis in ED or ICU has been reported consistently in many studies and validated for various health-care systems [19, 20]. Moreover, PCT testing can reduce long-term adverse events, 28-day mortality, and cost of hospitalization [30].
A consistent and correct application of the PCT-based algorithms for antibiotics treatment, as proposed in the consensus paper [10], will likely result in:
- Improved clinical management of patients with suspected bacterial infection such as sepsis
- Significant cost savings, primarily by reducing length of stay and duration of antibiotics therapy [19,20,30]
References+ View more
- Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263-273. doi:10.3343/alm.2014.34.4.263
- Jekarl DW, Lee S, Kim M, et al. Procalcitonin as a prognostic marker for sepsis based on SEPSIS-3. J Clin Lab Anal. 2019;33(9):e22996. doi:10.1002/jcla.22996
- Liu D, Su LX, Guan W, et al. Prognostic value of procalcitonin in pneumonia: A systematic review and meta-analysis. Respirology. 2016;21(2):280-288. doi:10.1111/resp.12704
- Kutz A, Briel M, Christ-Crain M, et al. Prognostic value of procalcitonin in respiratory tract infections across clinical settings. Crit Care. 2015;19(1):74. Published 2015 Mar 6. doi:10.1186/s13054-015-0792-1
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi:10.1001/jama.2016.0287
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008 [published correction appears in Crit Care Med. 2008 Apr;36(4):1394-6]. Crit Care Med. 2008;36(1):296-327. doi:10.1097/01.CCM.0000298158.12101.41
- Roope LSJ, Buchanan J, Morrell L, et al. Why do hospital prescribers continue antibiotics when it is safe to stop? Results of a choice experiment survey. BMC Med. 2020;18(1):196. Published 2020 Jul 30. doi:10.1186/s12916-020-01660-4
- Yu H, Nie L, Liu A, et al. Combining procalcitonin with the qSOFA and sepsis mortality prediction. Medicine (Baltimore). 2019;98(23):e15981. doi:10.1097/MD.0000000000015981
- Schuetz P, Bolliger R, Merker M, et al. Procalcitonin-guided antibiotic therapy algorithms for different types of acute respiratory infections based on previous trials. Expert Rev Anti Infect Ther. 2018;16(7):555-564. doi:10.1080/14787210.2018.1496331
- Schuetz P, Beishuizen A, Broyles M, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts’ consensus on optimized clinical use. Clin Chem Lab Med 2019 ;57 :1308-18
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596. doi:10.1097/01.CCM.0000217961.75225.E9
- Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463-474. doi:10.1016/S0140-6736(09)61879-1
- Sager R, Kutz A, Mueller B, Schuetz P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15(1):15. Published 2017 Jan 24. doi:10.1186/s12916-017-0795-7
- van Houten CB, Cohen A, Engelhard D, et al. Antibiotic misuse in respiratory tract infections in children and adults-a prospective, multicentre study (TAILORED Treatment). Eur J Clin Microbiol Infect Dis. 2019;38(3):505-514. doi:10.1007/s10096-018-03454-2
- Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 2014; 40: 32–40.
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and Safety of Procalcitonin Guidance in Reducing the Duration of Antibiotic Treatment in Critically Ill Patients: A Randomised, Controlled, Open-Label Trial. Lancet Infect Dis 2016; 16(7): 819-827.
- Schuetz P, Birkhahn R, Sherwin R, et al. Serial Procalcitonin Predicts Mortality in Severe Sepsis Patients: Results From the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit Care Med. 2017;45(5):781-789. doi:10.1097/CCM.0000000000002321
- Schuetz P, Wirz Y, Sager R, Christ-Grain M, Stolz D, Tamm M, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018; 18:95–107. https://doi.org/10.1016/S1473-3099(17)30592-3 PMID: 29037960
- Mewes JC, Pulia MS, Mansour MK, Broyles MR, Nguyen HB, Steuten LM. The cost impact of PCT-guided antibiotic stewardship versus usual care for hospitalised patients with suspected sepsis or lower respiratory tract infections in the US: A health economic model analysis. PLoS One. 2019;14(4):e0214222. Published 2019 Apr 23. doi:10.1371/journal.pone.0214222
- Voermans AM, Mewes JC, Broyles MR, Steuten LMG. Cost-Effectiveness Analysis of a Procalcitonin-Guided Decision Algorithm for Antibiotic Stewardship Using Real-World U.S. Hospital Data. OMICS. 2019;23(10):508-515. doi:10.1089/omi.2019.0113
- In: Meyler's Side Effects of Antimicrobial Drugs; 1st Edition (2010). Author: Jeffrey K. Aronso.
- Stojanovic I, Schneider JE, Wei L, Hong Z, Keane C, Schuetz P. Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: a Chinese hospital system perspective. Clin Chem Lab Med. 2017;55(4):561-570. doi:10.1515/cclm-2016-0349
- Schuetz P, Müller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;2012(9):CD007498. Published 2012 Sep 12. doi:10.1002/14651858.CD007498.pub2
- Schuetz Ph, Balk R, Briel M, et al. Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: a US health system perspective. Clin Chem Lab Med 2015; 53(4): 583–592
- Smith KJ, Wateska A, Nowalk MP, et al. Cost-effectiveness of procalcitonin-guided antibiotic use in community acquired pneumonia. J Gen Intern Med. 2013;28(9):1157-1164. doi:10.1007/s11606-013-2400-x
- Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of Procalcitonin Testing on Health-care Utilization and Costs in Critically Ill Patients in the United States. Chest. 2017;151(1):23-33. doi:10.1016/j.chest.2016.06.046
- Wilke MH, Grube RF, Bodmann KF. The use of a standardized PCT-algorithm reduces costs in intensive care in septic patients - a DRG-based simulation model. Eur J Med Res. 2011;16(12):543-548. doi:10.1186/2047-783x-16-12-543
- Kip MM, Kusters R, IJzerman MJ, Steuten LM. A PCT algorithm for discontinuation of antibiotic therapy is a cost-effective way to reduce antibiotic exposure in adult intensive care patients with sepsis. J Med Econ. 2015;18(11):944-953. doi:10.3111/13696998.2015.1064934
- Westwood M, Ramaekers B, Whiting P, et al. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2015;19(96):v-236. doi:10.3310/hta19960
- Kyriazopoulou E, Liaskou-Antoniou L, Adamis G, et al. Procalcitonin to Reduce Long-Term Infection-associated Adverse Events in Sepsis. A Randomized Trial. Am J Respir Crit Care Med. 2021 Jan 15;203(2):202-210. doi: 10.1164/rccm.202004-1201OC. PMID: 32757963; PMCID: PMC7874409.
References
- Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263-273. doi:10.3343/alm.2014.34.4.263
- Jekarl DW, Lee S, Kim M, et al. Procalcitonin as a prognostic marker for sepsis based on SEPSIS-3. J Clin Lab Anal. 2019;33(9):e22996. doi:10.1002/jcla.22996
- Liu D, Su LX, Guan W, et al. Prognostic value of procalcitonin in pneumonia: A systematic review and meta-analysis. Respirology. 2016;21(2):280-288. doi:10.1111/resp.12704
- Kutz A, Briel M, Christ-Crain M, et al. Prognostic value of procalcitonin in respiratory tract infections across clinical settings. Crit Care. 2015;19(1):74. Published 2015 Mar 6. doi:10.1186/s13054-015-0792-1
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi:10.1001/jama.2016.0287
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008 [published correction appears in Crit Care Med. 2008 Apr;36(4):1394-6]. Crit Care Med. 2008;36(1):296-327. doi:10.1097/01.CCM.0000298158.12101.41
- Roope LSJ, Buchanan J, Morrell L, et al. Why do hospital prescribers continue antibiotics when it is safe to stop? Results of a choice experiment survey. BMC Med. 2020;18(1):196. Published 2020 Jul 30. doi:10.1186/s12916-020-01660-4
- Yu H, Nie L, Liu A, et al. Combining procalcitonin with the qSOFA and sepsis mortality prediction. Medicine (Baltimore). 2019;98(23):e15981. doi:10.1097/MD.0000000000015981
- Schuetz P, Bolliger R, Merker M, et al. Procalcitonin-guided antibiotic therapy algorithms for different types of acute respiratory infections based on previous trials. Expert Rev Anti Infect Ther. 2018;16(7):555-564. doi:10.1080/14787210.2018.1496331
- Schuetz P, Beishuizen A, Broyles M, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts’ consensus on optimized clinical use. Clin Chem Lab Med 2019 ;57 :1308-18
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596. doi:10.1097/01.CCM.0000217961.75225.E9
- Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463-474. doi:10.1016/S0140-6736(09)61879-1
- Sager R, Kutz A, Mueller B, Schuetz P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15(1):15. Published 2017 Jan 24. doi:10.1186/s12916-017-0795-7
- van Houten CB, Cohen A, Engelhard D, et al. Antibiotic misuse in respiratory tract infections in children and adults-a prospective, multicentre study (TAILORED Treatment). Eur J Clin Microbiol Infect Dis. 2019;38(3):505-514. doi:10.1007/s10096-018-03454-2
- Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 2014; 40: 32–40.
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and Safety of Procalcitonin Guidance in Reducing the Duration of Antibiotic Treatment in Critically Ill Patients: A Randomised, Controlled, Open-Label Trial. Lancet Infect Dis 2016; 16(7): 819-827.
- Schuetz P, Birkhahn R, Sherwin R, et al. Serial Procalcitonin Predicts Mortality in Severe Sepsis Patients: Results From the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit Care Med. 2017;45(5):781-789. doi:10.1097/CCM.0000000000002321
- Schuetz P, Wirz Y, Sager R, Christ-Grain M, Stolz D, Tamm M, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018; 18:95–107. https://doi.org/10.1016/S1473-3099(17)30592-3 PMID: 29037960
- Mewes JC, Pulia MS, Mansour MK, Broyles MR, Nguyen HB, Steuten LM. The cost impact of PCT-guided antibiotic stewardship versus usual care for hospitalised patients with suspected sepsis or lower respiratory tract infections in the US: A health economic model analysis. PLoS One. 2019;14(4):e0214222. Published 2019 Apr 23. doi:10.1371/journal.pone.0214222
- Voermans AM, Mewes JC, Broyles MR, Steuten LMG. Cost-Effectiveness Analysis of a Procalcitonin-Guided Decision Algorithm for Antibiotic Stewardship Using Real-World U.S. Hospital Data. OMICS. 2019;23(10):508-515. doi:10.1089/omi.2019.0113
- In: Meyler's Side Effects of Antimicrobial Drugs; 1st Edition (2010). Author: Jeffrey K. Aronso.
- Stojanovic I, Schneider JE, Wei L, Hong Z, Keane C, Schuetz P. Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: a Chinese hospital system perspective. Clin Chem Lab Med. 2017;55(4):561-570. doi:10.1515/cclm-2016-0349
- Schuetz P, Müller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;2012(9):CD007498. Published 2012 Sep 12. doi:10.1002/14651858.CD007498.pub2
- Schuetz Ph, Balk R, Briel M, et al. Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: a US health system perspective. Clin Chem Lab Med 2015; 53(4): 583–592
- Smith KJ, Wateska A, Nowalk MP, et al. Cost-effectiveness of procalcitonin-guided antibiotic use in community acquired pneumonia. J Gen Intern Med. 2013;28(9):1157-1164. doi:10.1007/s11606-013-2400-x
- Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of Procalcitonin Testing on Health-care Utilization and Costs in Critically Ill Patients in the United States. Chest. 2017;151(1):23-33. doi:10.1016/j.chest.2016.06.046
- Wilke MH, Grube RF, Bodmann KF. The use of a standardized PCT-algorithm reduces costs in intensive care in septic patients - a DRG-based simulation model. Eur J Med Res. 2011;16(12):543-548. doi:10.1186/2047-783x-16-12-543
- Kip MM, Kusters R, IJzerman MJ, Steuten LM. A PCT algorithm for discontinuation of antibiotic therapy is a cost-effective way to reduce antibiotic exposure in adult intensive care patients with sepsis. J Med Econ. 2015;18(11):944-953. doi:10.3111/13696998.2015.1064934
- Westwood M, Ramaekers B, Whiting P, et al. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2015;19(96):v-236. doi:10.3310/hta19960
- Kyriazopoulou E, Liaskou-Antoniou L, Adamis G, et al. Procalcitonin to Reduce Long-Term Infection-associated Adverse Events in Sepsis. A Randomized Trial. Am J Respir Crit Care Med. 2021 Jan 15;203(2):202-210. doi: 10.1164/rccm.202004-1201OC. PMID: 32757963; PMCID: PMC7874409.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars







