Printed from acutecaretesting.org
January 2004
Useful tips to avoid preanalytical errors in blood gas testing: metabolites
Introduction
For the purpose of this article, a preanalytical error is defined as a deviating result caused by one of the following steps in the preanalytical phase:
- Patient preparation
- Blood sampling
- Sample handling
- Sample transport and storage
This article focuses on preanalytical issues concerning the metabolite parameters available on a modern blood gas analyzer (cGlu and cLac) when measured on an arterial whole-blood sample together with blood gas parameters. However, the focus of the article is not restricted to this application only, as metabolites are also measured on venous blood, capillary blood, plasma and serum.
Analytical factors causing a deviation in results and biological variations are not dealt with in this article.
The article starts by giving a general description of the main reasons behind preanalytical errors when measuring metabolites and the biochemical background of their influence on measurements.
Next, it provides lists with useful tips on how to avoid preanalytical errors when measuring metabolite parameters on blood gas analyzers. The lists can be used as checklists when a specific problem is encountered, or as a tool to supplement or expand the knowledge of the staff involved in laboratory medicine, e.g. when updating procedures or when conducting refresher training.
Preanalytical issues – main reasons of preanalytical errors
The three main reasons of preanalytical errors when measuring metabolites on a blood gas analyzer are:- Storage time
- Storage temperature
- Abnormal cell count
Storage time
The glycolysis continues in whole blood after sampling. This means that the cells continue to metabolize glucose with the purpose of producing energy for the cellular functions. The glycolysis is a metabolic pathway that converts one glucose molecule into two molecules of pyruvate. Depending of the type of cell and the availability of oxygen, pyruvate will be further metabolized by one of two pathways.
If oxygen is present (aerobic metabolism), pyruvate will be oxidized to CO2 and H2O in a regulated process that creates energy (see Fig. 1). Under anaerobic conditions and in the erythrocytes, pyruvate will be reduced to lactate; a process that creates much less energy (see Fig. 2). This means that the glycolysis will consume glucose and produce lactate in a whole-blood sample stored for a longer period of time
.
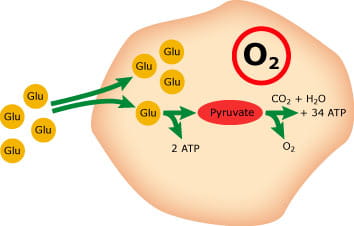
FIG. 1: Aerobic metabolism
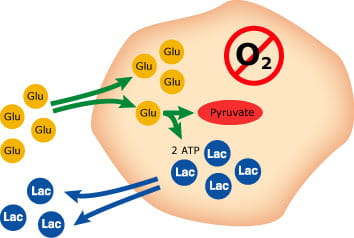
FIG. 2: Anaerobic metabolism
Example:
The rate of change of cGlu and cLac at room temperature (22-25 °C (72-77 °F)) found in various studies are presented in the table below. All studies are based upon samples with values within the reference range:
|
Rate of |
Rate of |
Rate of change |
Reference |
|
|
cGlu |
-0.5 |
-9 |
10 % |
[1] |
|
-0.5 |
-9 |
10 % |
[2] |
|
|
-0.3 |
-5 |
6 % |
[4] |
|
|
-0.4 |
-6 |
8 % |
[9] |
TABLE I
|
Rate of |
Rate of |
Rate of change |
Reference |
|
|
cLac |
+0.6 |
+5 |
57 % |
[1] |
|
+0.6 |
+5 |
57 % |
[4] |
|
|
+0.4 |
+4 |
38 % |
[10] |
|
|
+0.4 |
+4 |
38 % |
[11] |
TABLE II
*Normal value is calculated as the mean reference value [3].
Reference values
cGlu(fPt)(aP):
- 3.9-5.8 mmol/L
- 70-105 mg/dL
cLac(aP):
- 0.5-1.6 mmol/L
- 4.5-14.4 mg/dL
fPt = fasting patient
aP = arterial plasma
Based on Tables I and II, it can be concluded that the average rate of change per hour for cGlu and cLac at room temperature is –0.40 mmol/L/h and 0.50 mmol/L/h, respectively.
Storage temperature
The on-going in vitro glycolysis in samples that cannot be measured immediately can be avoided or reduced to a minimum by different procedures. Two common methods are to add an antiglycolytic agent, such as a fluoride salt, to the sample, or simply to store the sample at or below 4 °C (34 °F).
However, fluoride salts only have a significant effect after a minimum of one hour [12,13,14,15] of storage and may even have a hemolytic effect on the erythrocytes [1,16]. Besides, fluoride is not compatible with the biosensors used for glucose and lactate determinations in blood gas analyzers [17].
Immediate analysis (defined by Kost et al [1] to be within 15 minutes after the sample has been taken) or cool storage are the preferred and easiest options for avoiding glycolysis. Storage at cool temperatures slows down the glycolytic processes, having a significant effect on especially lactate.
Example:
The influence of storage temperature on lactate (Table III) and glucose (Table IV) found in various studies are presented in the table below. All studies are based upon samples with values within the normal range:
|
cLac (mmol/L) changes after one hour of storage |
Reference |
|
|
At 4°C (34 °F) |
At room temperature |
|
|
+0.06 |
+0.63 |
[4] |
|
+0.4 |
+0.4 |
[10] |
|
+0.09 |
+0.42 |
[11] |
|
+0.21 |
+0.72 |
[18] |
|
+0.12 |
+0.45 |
[19] |
|
Mean: 0.18 mmol/L |
Mean: 0.52 mmol/L |
|
TABLE III
|
cGlu (mmol/L) changes after one hour of storage |
Reference |
|
|
At 4°C (34 °F) |
At room temperature |
|
|
-0.03 |
-0.32 |
[4] |
TABLE IV
There is a huge discrepancy in the level of changes shown in the various studies concerning lactate. The reason may be that different measuring and preparation methods have been used. However, the effect of storage temperature on cLac and cGlu cannot be questioned!
3. Abnormal cell count
The glycolysis continues in vitro, because of the presence of energy-consuming cells in the sample. The cells continue to metabolize glucose to produce energy for various cellular functions, e.g. the Na-K pump [20]. The cells are divided into three main groups and their numbers are determined by a complete blood cell count (CBC):
- Erythrocytes or red blood cells
- Thrombocytes or platelets
- Leukocytes or white blood cells
An abnormally high blood cell count will influence the rate of glycolysis and thereby the stability of cGlu and cLac over time. Especially leukocytosis [18] but also an increase of erythrocytes (high hematocrit) [13,15,21] have been reported to affect the rate of the glycolysis.
Example:
The influence of leukocytosis (Table V) and high hematocrit (Table VI) on cLac and cGlu at room temperature is often mentioned in the literature [1,10,18,22,13,15,21]. However, only few studies present actual data. All studies in Tables V and VI are based upon samples with values within the normal range:
|
Leukocyte count |
Rate of change of |
Reference |
|
23–53 x 109 cells/L |
+0.8* |
[10] |
|
> 60 x 109 cells/L |
+0.8* |
[18] |
TABLE V
*This value should be compared with the average rate of change for cLac at room temperature with a normal cell count (refer to Tables II and III) at +0.5 mmol/L/h.|
Hematocrit % |
Rate of change of |
Reference |
|
35 % |
11 % |
[2] |
|
55 % |
19 % |
[2] |
|
43 % |
7 % |
[15] |
|
75 % |
12 % |
[15] |
TABLE VI
Reference values [23]
Leukocytes:
- 4.5–11 x 109 cells/L
Hematocrit:
- (male) 42-52 %
- (female) 35-47 %
3.1 Neonatal blood
Neonatal blood is known to have a very high hematocrit level and is therefore expected to have a higher rate of glycolysis [14,16,15,24]. There is some discrepancy in the reporting of the rate of glycolysis in neonatal blood. A recent study [24] showed a rate of change for glucose levels in neonate blood of –0.36 mmol/L/h (~ 8 %), which is very similar to the rate of change for adult blood (~-0.40 mmol/L/h).
However, an older study [14] showed a rate of change per hour of 23.7 %. In any case, it must be emphasized that the changes are significant for newborn infants with the risk of hypoglycemia.
Storage conditions – conclusion
The effect of storage time on cGlu and cLac in whole blood depends on time, temperature, blood cell count, pO2 and tHb [1,10,18]. However, if the general recommendation for storage of samples for blood gas measurement is followed, the influence of these factors will be negligible [18].
Useful tips
Below you will find detailed lists of preanalytical issues affecting cGlu and cLac. The information found in the lists is based on observations reported in various works relating to the preanalytical phase. Some of the observations have a larger impact on the measurement result than others. Nevertheless, no observation should be ignored.The lists can be used as checklists for training in the different steps of sampling, or whenever a specific problem is encountered during:
- Patient preparation
- Blood sampling
- Sample handling
- Sample transport and storage
Useful tips when measuring glucose (cGlu)
|
Make sure that: |
To avoid: |
|
Patient preparation |
|
|
The sample is not drawn from a patient to whom an infusion solution containing glucose has been given within the last hour [16,25] |
cGlu |
|
Blood sampling |
|
|
The sample type is specified when reporting the result, as there is a difference between arterial, venous and capillary blood [26,27,28] |
cGlu |
|
Sample handling |
|
|
Samples containing fluoride [11] as a stabilizer are not measured on a blood gas analyzer |
cGlu |
|
Sample transport and storage |
|
|
Samples that are not measured immediately are stored at 0-4 °C (32-39 °F) |
cGlu |
Useful tips when measuring lactate (cLac)
|
Make sure that: |
To avoid: |
|
Patient preparation |
|
|
The sample is not drawn from a patient to whom an infusion solution containing lactate has been given within the last hour [16,25] |
|
|
The patient has not exercised prior to the sampling [16,29] |
cLac |
|
Blood sampling |
|
|
The venous stasis is released after max. one minute to avoid anaerobic metabolism [4,16,25] |
cLac |
|
The sample type is specified when reporting the result, as there is a difference between arterial, venous and capillary blood [30,31] |
cLac |
|
Sample handling |
|
|
The sample is not hemolyzed, as this will release intracellular lactate dehydrogenase, which promotes the conversion from pyruvate to lactate [2] |
cLac |
|
Samples containing fluoride [11] as a stabilizer are not measured on a blood gas analyzer |
|
|
Sample transport and storage |
|
|
Samples that are not measured immediately are stored at 0-4 °C (32-39 °F) |
cLac |
Discussion
Storage time and temperature are the two most important preanalytical issues to be aware of when measuring cGlu and cLac. If the samples are stored correctly, or as generally recommended for blood gas samples, the rate of glycolysis will not influence the measurement result even if the sample has an abnormal blood cell count. However, if the storage time is prolonged, all of these factors will have a significant influence on the measurement result.Other issues that are important to mention here, even though they are analytical problems rather than preanalytical, are to know what is measured and what is reported.
There are a number of different measuring methods available for cGlu and cLac, e.g.:
- Biosensors on blood gas analyzers
- POC glucometers
- Chemistry analyzers
The different methods measure on different specimen types, such as the plasma phase of whole blood, whole blood, plasma or hemolysate. It can be difficult to distinguish between preanalytical errors and analytical variations that come from different measuring techniques [32,33] – especially if the different methods are compared with one another. To learn more about different ways of reporting glucose results, read [34].
Conclusion
Measurement of plasma glucose is the single most frequent measured parameter in laboratory medicine today, while lactate is becoming an important prognostic parameter in intensive care.
This article has described the three main causes of preanalytical errors related to measurements on these two parameters and provided tips that will help you prevent errors when measuring these parameters on blood gas analyzers.
References+ View more
- Kost GJ, Nguyen TH, Tang Z. Whole-blood glucose and lactate. Arch Pathol Lab 2000; 124: 1128-34.
- Young DS. Effects of preanalytical variables on clinical laboratory test. 2nd ed. Washington, DC: AACC Press, 1997.
- Reference Manual for the ABL700 Series. Ed. I. Radiometer publication, Copenhagen. Radiometer Medical A/S, 2003.
- Andersen O, Haugaard SB, Jørgensen LT et al. Preanalytical handling of samples for measurement of plasma lactate in HIV patients. Scand J Clin Lab Invest 2003; 63, 449-54.
- van den Berghe G, Wouters P, Weekers F et al. Intensive insulin therapy in critically ill patients. The New England Journal of Medicine 2001; 345, 1359-67.
- Toffaletti J. Elevations in blood lactate: overview of use in critical care. Scand J Clin Lab Invest 1996; 56: 107-10.
- Bakker J. Increased blood lactate levels: A marker of…? http://www.acutecaretesting.org/, 2003.
- Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care 2003; 26: 917-32.
- Miller SJ, Wallace RJ, Musher DM et al. Hypoglycemia as a manifestation of sepsis. Am J Med 1980; 68: 649-54.
- Astles R, Williams C, Sedor F. Stability of plasma lactate in vitro in the presence of antiglycolytic agents. Clin Chem 1994; 40, 7: 1327-30.
- Wandrup J, Tvede K, Grinsted J, Jordening H. "Stat" measurement of L-lactate in whole blood and cerebrospinal fluid assessed. Clin Chem 1989; 35, 8: 1740-43.
- Chan AYW, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem 1989; 35, 2: 315-17.
- de Pasqua A, Mattock MB, Phillips R, Keen H. Errors in blood glucose determination. The Lancet 1984; 17: 1165.
- Meites S, Saniel-Banrey K. Preservation, distribution, and assay of glucose in blood, with special reference to the newborn. Clin Chem 1979; 25, 4: 531-34.
- Sidebottom RA, Williams PR, Kanarek KS. Glucose determinations in plasma and serum: potential error related to increased hematocrit. Clin Chem 1982; 28: 190-92.
- Narayanan S. The preanalytical phase – an important component of laboratory medicine. Am J Clin Pathol 2000; 113, 429-52.
- Wandrup JH, Walcker K. New improvements of stat-lactate measurements on whole blood. Radiometer Publication AS123. Copenhagen: Radiometer Medical A/S, 1997.
- Calatayud O, Tenias JM. Effects of time, temperature and blood cell counts on levels of lactate in heparinized whole blood gas samples. Scand J Clin Lab Invest 2003; 63: 311-14.
- Chariot P, Ratiney R, Ammi-Said M, Herigault R, Adnot S, Gherardi R. Optimal handling of blood samples for routine measurement of lactate and pyruvate. Arch Pathol Lab Med 1994; 118, 695-97.
- Wennecke G. Useful tips to avoid preanalytical errors in blood gas testing: electrolytes. http://www.acutecaretesting.org/, 2003.
- Ladenson JH. Nonanalytical sources of variation in clinical chemistry results. In: Sonnenwirth AC, Jarrett L. Gradwohl's clinical laboratory methods and diagnosis. St. Louis: C.V. Mosby Co., 1980: 149–92.
- Fleisher M, Gladstone M, Schwartz M, Crystal D. The whole-blood multi-analyte analyzers evaluated. Clin Chem 1989; 35, 7: 1532-35.
- Jacobs DS, DeMott WR, Oxley DK. Laboratory test handbook. 5th ed. Hudson, OH: Lexi-Comp Inc., 2001.
- Bjurström K, Fridolf T, Andréasson B. Glycolysis in neonatal blood decreases glucose concentration by 0.36 mmol/h. Clin Chim Acta 2001; 307: 259.
- Müller-Plathe O. Preanalytical aspects in STAT analysis. Blood Gas News 1998; 7, 1: 4-11.
- Stahl M, Brandslund I, Jørgensen LGM, Hyltoft Petersen P, Borch-Johnsen K, de Fine Olivarius N. Can capillary whole blood glucose and venous plasma glucose measurements be used interchangeably in diagnosis of diabetes mellitus? Scand J Clin Lab Invest 2002; 62, 2: 159-66.
- Stahl M, Brandslund I. Measurement of glucose content in plasma from capillary blood in diagnosis of diabetes mellitus. Scand J Clin Lab Invest 2003; 63: 431-40.
- Juretic D, Bozicevic S, Lovrencic MV. Pre-analytical, analytical and post-analytical factors influencing specific tests for diagnosis and monitoring of DM-national network in quality assessment. eJIFCC 2002; 13, 5: 1-5.
- Harris R, Dudley G. Exercise alters the distribution of ammonia and lactate in blood. J Appl Physical 1988; 66,1: 313-17.
- Williams JR Armstrong N, Kirby BJ. The influence of the site of sampling and assay medium upon the measurement and interpretation of blood lactate responses to exercise. Journal of Sports Sciences 1990; 10, 95-107.
- Foxdal P, Sjödin B, Rudstam H, Östman C, Östman B, Hedenstierna G. Lactate concentration differences in plasma, whole blood, capillary finger blood and erythrocytes during submaximal graded exercise in humans. Eur J Appl Physiol 1989; 61, 218-22.
- Wiener K. Principles and problems of blood glucose measurement. http://www.acutecaretesting.org/ 2003
- Tofaletti J, Hammes ME, Gray R, Lineberry B, Abrams B. Lactate measured in diluted and undiluted whole blood and plasma: Comparison of methods and effect of hematocrit. Clin Chem 1992; 38, 12: 2430-34.
- Skurup A. Glucose measuring system – what is measured and what is reported? Radiometer Publication Bulletin No. 8. Copenhagen: Radiometer Medical A/S, 1998.
References
- Kost GJ, Nguyen TH, Tang Z. Whole-blood glucose and lactate. Arch Pathol Lab 2000; 124: 1128-34.
- Young DS. Effects of preanalytical variables on clinical laboratory test. 2nd ed. Washington, DC: AACC Press, 1997.
- Reference Manual for the ABL700 Series. Ed. I. Radiometer publication, Copenhagen. Radiometer Medical A/S, 2003.
- Andersen O, Haugaard SB, Jørgensen LT et al. Preanalytical handling of samples for measurement of plasma lactate in HIV patients. Scand J Clin Lab Invest 2003; 63, 449-54.
- van den Berghe G, Wouters P, Weekers F et al. Intensive insulin therapy in critically ill patients. The New England Journal of Medicine 2001; 345, 1359-67.
- Toffaletti J. Elevations in blood lactate: overview of use in critical care. Scand J Clin Lab Invest 1996; 56: 107-10.
- Bakker J. Increased blood lactate levels: A marker of…? http://www.acutecaretesting.org/, 2003.
- Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care 2003; 26: 917-32.
- Miller SJ, Wallace RJ, Musher DM et al. Hypoglycemia as a manifestation of sepsis. Am J Med 1980; 68: 649-54.
- Astles R, Williams C, Sedor F. Stability of plasma lactate in vitro in the presence of antiglycolytic agents. Clin Chem 1994; 40, 7: 1327-30.
- Wandrup J, Tvede K, Grinsted J, Jordening H. "Stat" measurement of L-lactate in whole blood and cerebrospinal fluid assessed. Clin Chem 1989; 35, 8: 1740-43.
- Chan AYW, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem 1989; 35, 2: 315-17.
- de Pasqua A, Mattock MB, Phillips R, Keen H. Errors in blood glucose determination. The Lancet 1984; 17: 1165.
- Meites S, Saniel-Banrey K. Preservation, distribution, and assay of glucose in blood, with special reference to the newborn. Clin Chem 1979; 25, 4: 531-34.
- Sidebottom RA, Williams PR, Kanarek KS. Glucose determinations in plasma and serum: potential error related to increased hematocrit. Clin Chem 1982; 28: 190-92.
- Narayanan S. The preanalytical phase – an important component of laboratory medicine. Am J Clin Pathol 2000; 113, 429-52.
- Wandrup JH, Walcker K. New improvements of stat-lactate measurements on whole blood. Radiometer Publication AS123. Copenhagen: Radiometer Medical A/S, 1997.
- Calatayud O, Tenias JM. Effects of time, temperature and blood cell counts on levels of lactate in heparinized whole blood gas samples. Scand J Clin Lab Invest 2003; 63: 311-14.
- Chariot P, Ratiney R, Ammi-Said M, Herigault R, Adnot S, Gherardi R. Optimal handling of blood samples for routine measurement of lactate and pyruvate. Arch Pathol Lab Med 1994; 118, 695-97.
- Wennecke G. Useful tips to avoid preanalytical errors in blood gas testing: electrolytes. http://www.acutecaretesting.org/, 2003.
- Ladenson JH. Nonanalytical sources of variation in clinical chemistry results. In: Sonnenwirth AC, Jarrett L. Gradwohl's clinical laboratory methods and diagnosis. St. Louis: C.V. Mosby Co., 1980: 149–92.
- Fleisher M, Gladstone M, Schwartz M, Crystal D. The whole-blood multi-analyte analyzers evaluated. Clin Chem 1989; 35, 7: 1532-35.
- Jacobs DS, DeMott WR, Oxley DK. Laboratory test handbook. 5th ed. Hudson, OH: Lexi-Comp Inc., 2001.
- Bjurström K, Fridolf T, Andréasson B. Glycolysis in neonatal blood decreases glucose concentration by 0.36 mmol/h. Clin Chim Acta 2001; 307: 259.
- Müller-Plathe O. Preanalytical aspects in STAT analysis. Blood Gas News 1998; 7, 1: 4-11.
- Stahl M, Brandslund I, Jørgensen LGM, Hyltoft Petersen P, Borch-Johnsen K, de Fine Olivarius N. Can capillary whole blood glucose and venous plasma glucose measurements be used interchangeably in diagnosis of diabetes mellitus? Scand J Clin Lab Invest 2002; 62, 2: 159-66.
- Stahl M, Brandslund I. Measurement of glucose content in plasma from capillary blood in diagnosis of diabetes mellitus. Scand J Clin Lab Invest 2003; 63: 431-40.
- Juretic D, Bozicevic S, Lovrencic MV. Pre-analytical, analytical and post-analytical factors influencing specific tests for diagnosis and monitoring of DM-national network in quality assessment. eJIFCC 2002; 13, 5: 1-5.
- Harris R, Dudley G. Exercise alters the distribution of ammonia and lactate in blood. J Appl Physical 1988; 66,1: 313-17.
- Williams JR Armstrong N, Kirby BJ. The influence of the site of sampling and assay medium upon the measurement and interpretation of blood lactate responses to exercise. Journal of Sports Sciences 1990; 10, 95-107.
- Foxdal P, Sjödin B, Rudstam H, Östman C, Östman B, Hedenstierna G. Lactate concentration differences in plasma, whole blood, capillary finger blood and erythrocytes during submaximal graded exercise in humans. Eur J Appl Physiol 1989; 61, 218-22.
- Wiener K. Principles and problems of blood glucose measurement. http://www.acutecaretesting.org/ 2003
- Tofaletti J, Hammes ME, Gray R, Lineberry B, Abrams B. Lactate measured in diluted and undiluted whole blood and plasma: Comparison of methods and effect of hematocrit. Clin Chem 1992; 38, 12: 2430-34.
- Skurup A. Glucose measuring system – what is measured and what is reported? Radiometer Publication Bulletin No. 8. Copenhagen: Radiometer Medical A/S, 1998.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Minimizing Pre-analytical errors in blood gas testing
Presented by Ana-Maria Simundic, PhD, Prof. of Medical Biochemistry, Zagreb University, Zagreb, Croatia Watch the webinarRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinarRelated webinar
Evolution of blood gas testing Part 2
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine. Watch the webinarBlood gas Preanalytics app
Get the Blood gas Preanalytics app for your smartphone
This smartphone app focuses on the preanalytical phase of blood gas testing and what operators can do to avoid errors. Download appScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars