Printed from acutecaretesting.org
January 2015
Why D-dimer tests cannot be used to exclude venous thromboembolism in patients with high pretest probability
SummaryD-dimer testing can combined with assessment of pretest probability be used to rule out VTE in patients with a sufficiently low pretest probability of VTE. Patients with a negative D-dimer result can be ruled out, while patients with a positive D-dimer result need to have imaging performed for confirmatory diagnosis.
However, D-dimer testing cannot be used in patients assessed to have a high pretest probability. The reason for this is that the prevalence of VTE in this group is high and as the negative predictive value is dependent on prevalence, too many sick individuals would wrongly be ruled out if D-dimer testing was used in the high-pretest-probability group.
For the safety of the patients this should always be kept in mind – a D-dimer test cannot be used for rule out in high-pretest-probability patients.
GLOSSARY AND ABBREVIATIONS
Abbreviation/term |
Meaning |
| DVT | Deep vein thrombosis |
| FDA | US Food and Drug Administration |
| NPV | Negative predictive value, fraction of test-negatives who do not have disease |
| PE | Pulmonary embolism |
| PPV | Positive predictive value, fraction of test-positives who do have disease |
| Prevalence | Fraction of persons with disease in the tested population |
| Sens | Sensitivity, fraction of sick persons getting a result above cut-off |
| Spec | Specificity, fraction of healthy persons getting a result below cut-off |
| VTE | Venous thromboembolism (includes DVT and PE) |
INTRODUCTION
Venous thromboembolism (VTE) refers to disease states that include both deep venous thrombosis (DVT) and pulmonary embolism (PE).
Many clinical societies have made official guidelines for diagnosis of DVT [1] and for diagnosis of PE[2]. In addition, the CLSI (Clinical and Laboratory Standard Institute) has a guideline for the exclusion of venous thromboembolism [3].
All the guidelines agree that VTE diagnosis can be based on pretest probability and D-dimer testing for patients with a sufficiently low probability of VTE:
- Negative D-dimer result – Rule out the patient
- Positive D-dimer result – Perform imaging for confirmatory diagnosis
Even though all international and local guidelines on VTE diagnosis agree that in patients with a sufficiently low pretest probability, D-dimer testing can be used to rule out VTE and that patients with a high pretest probability should either receive confirmatory imaging at once, and if that is not possible, they should be treated at once [1, 2, 3], it is not in these guidelines explicitly explained why D-dimer cannot be used to rule out VTE in high-pretest-probability patients.
This paper will explain and graphically illustrate why D-dimer cannot be used to rule out VTE in high-pretest-probability patients.
The pretest probability, also called the clinical probability score, is the probability that a person has a particular disease/condition before any test results are obtained. The pretest probability for large groups of people (such as the population of a country) is the same as the prevalence of the disease in that group.
A flowchart of the diagnostic protocol can look like Fig. 1.
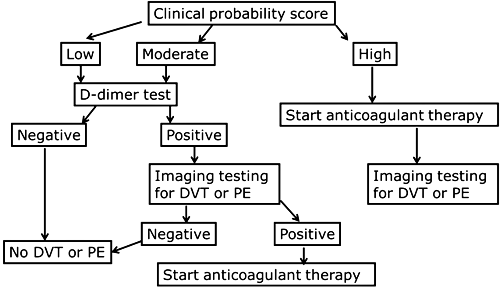
FIG. 1: Flowchart for diagnosis of deep vein thrombosis (DVT) or pulmonary embolism (PE) [1, 2]
The pretest determination is based on clinical information and algorithms. There are several scoring templates available like the Wells score and the Geneva score. The original scorings included three levels: ”low”, ”moderate” or ”high” probability. The scorings have been simplified to include two levels: ”unlikely” or ”likely”.
Patients with ”high”, ”DVT likely” or ”PE likely” pretest probabilities should either receive confirmatory imaging at once, and if that is not possible, they should be treated at once.
Patients with ”low”, ”moderate”, ”DVT unlikely” or ”PE unlikely” probabilities are tested with D-dimer. A negative D-dimer result means that DVT or PE can be ruled out. A positive D-dimer result means that the patient has to undergo further imaging in order to diagnose whether or not he or she has DVT or PE. This makes ruling out the main purpose of the D-dimer test. A test used for ruling out needs to have a high negative predictive value so that we can be sure that the patients we rule out are not sick.
INFLUENCE OF PREVALENCE ON THE NEGATIVE PREDICTIVE VALUE
The predictive value of a test is a measure in percentage of the times that the value (positive or negative) is the true value, i.e. the percentage of all with a positive test who are actually sick is the positive predictive value (PPV) and the percentage of all with a negative tests who are not sick is the negative predictive value (NPV).
The predictive values depend on the sensitivity and specificity of the test. However, the prevalence, i.e. the fraction of sick persons in the population tested, also has a strong impact on the predictive values.
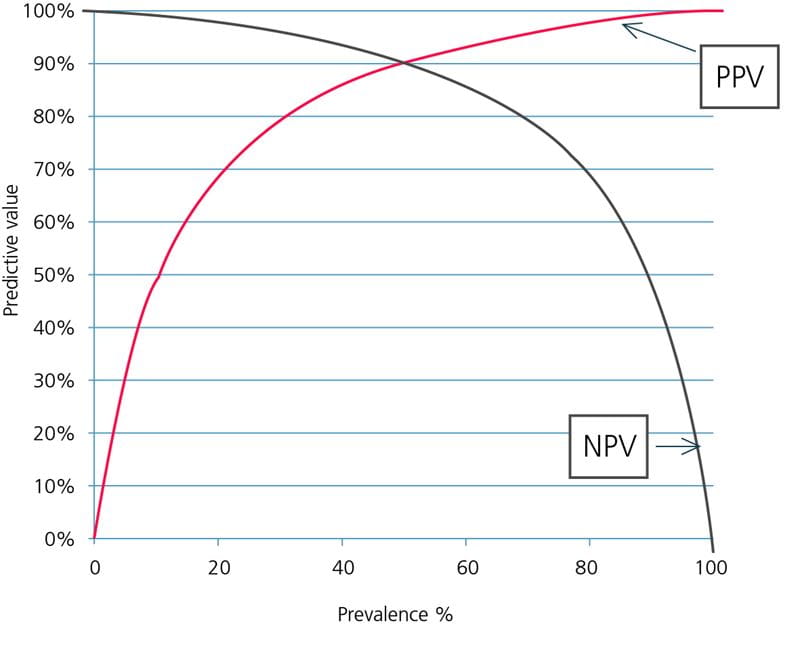
FIG. 2: Predictive values as function of prevalence. Assay sensitivity = 90 %, specificity = 90 %.
As can be seen in Fig. 2, the NPV decreases with increasing prevalence of disease and PPV increases with increasing prevalence of disease. To understand why that is, consider two populations:
-
No one in the population is sick (prevalence = 0 %). When there are only healthy persons in a population, every negative result is a true-negative result and the NPV is 100 %.
Every positive result is a false-positive result and the PPV is 0 %.
-
Everyone in a population is sick (prevalence = 100 %). When there are only sick persons in a population, every negative result is a false-negative result and the NPV is 0 %.
Every positive result is a true-positive result and the PPV is 100 %.
Thus we can see that the prevalence of disease impacts the PPV by influencing the true-positive and the false-positive rates and the NPV by influencing the true-negative and the false-negative rates.
As the negative predictive value is the major concern for a D-dimer test, I will focus on that in the following.
WHY D-DIMER TESTS SHOULD ONLY BE USED FOR PATIENTS WITH LOW/MODERATE/UNLIKELY PRETEST PROBABILITY
The assay sensitivity has a strong influence on the negative predictive value. This is because lowering the sensitivity mainly means increasing the number of false-negative results; see Fig. 3.
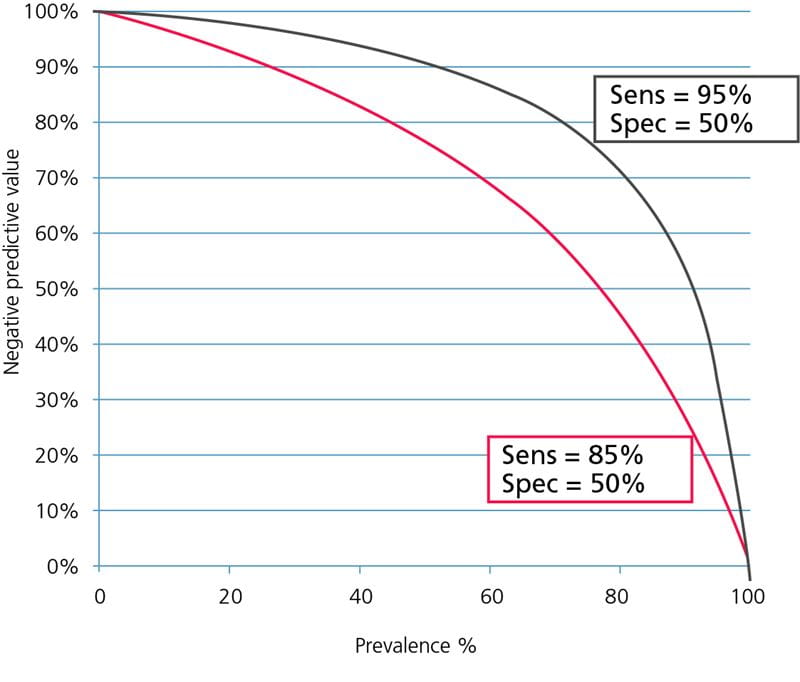
FIG. 3: Influence of sensitivity on the negative predictive value. Assay specificity = 50 %.
The influence of specificity on the negative predictive value is minimal because lowering the specificity mainly means increasing the number of false-positive results; see Fig. 4.
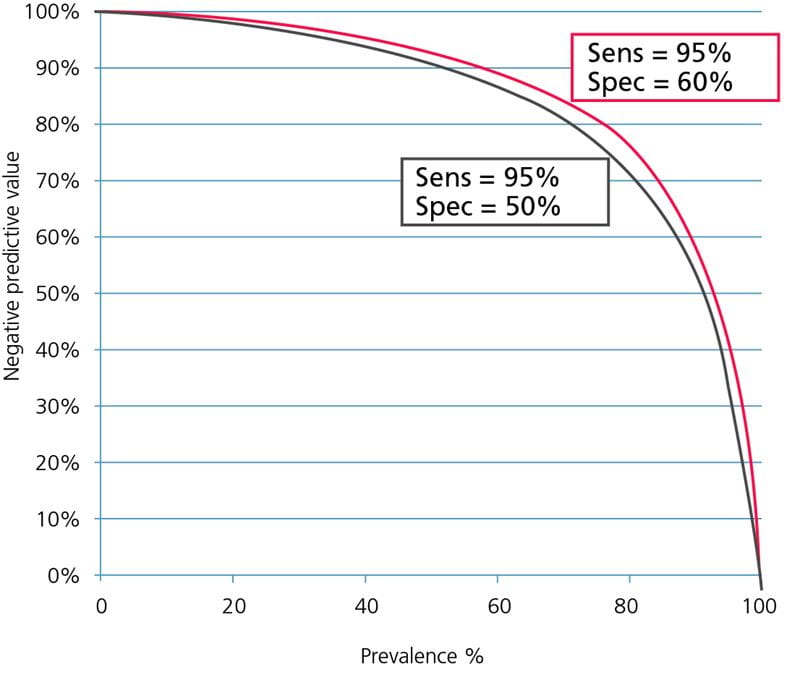
FIG. 4: Influence of specificity on the negative predictive value. Assay sensitivity = 95 %.
In the guidelines for diagnosing DVT and PE the D-dimer assays, which can be used in diagnosis, are grouped into [1, 2]:
- Highly sensitive assays. Diagnostic sensitivity ≥ 95 %.
Can be used to exclude DVT or PE in patients with either ”low”, ”moderate”, ”DVT unlikely” or ”PE unlikely” pretest probability.
- Moderately sensitive assays. Diagnostic sensitivity < 95 %.
Can be used to exclude DVT or PE in patients with either ”low”, ”DVT unlikely” or ”PE unlikely” pretest probability.
Whole-blood agglutination point-of-care D-dimer assays are known to have sensitivities of ~85 % while ELISA assays have sensitivities of ~95 % [4]. Specificities differ greatly between D-dimer assays but can typically be in the range of 30-70 % [5]. Therefore the following is based on comparison of the NPV as a function of prevalence for two hypothetical assays, which we will call Assay95 and Assay85:
- Assay95
- Sensitivity 95 % (the lower sensitivity limit in the high-sensitivity group of D-dimer assays)
- Specificity 50 %
- Assay85
- Sensitivity 85 % (a typical sensitivity in the moderate-sensitivity group of D-dimer assays)
- Specificity 50 %.
Now, why should D-dimer tests not be used to exclude venous thromboembolism in patients with ”high” pretest probability? To understand that we need to realize what a negative predictive value tells us.
If the NPV is 95 %, we know that in 95 % of the cases when we have a negative result the patient is not sick. Then we also know that in 5 % of the cases when we have a negative result the patient is actually sick.
If we dismiss all with a negative result and the NPV is 95 %, then 5 % of all those we dismiss based on a negative result will be sick. In our case, where we talk about patients suspected of venous thromboembolism, the consequences of misdiagnosis can be serious and include fatal pulmonary embolism.
What is an acceptable NPV? Preferably it should be 100 % but that is not always possible. The US Food and Drug Administration (FDA) require different levels of clearance for D-dimer assays dependent on whether the claim is intended use for ”aid in diagnosis of VTE” or for ”exclusion of VTE” [3].
However, regarding NPV the FDA requirement is the same for both claims: NPV ≥ 97 %. When this requirement is achieved, 3 % or less of those who are dismissed based on a negative D-dimer result will be sick.
The guideline for diagnosing DVT from the American College of Chest Physicians [1] estimates that the prevalence of DVT in the ”low”-pretest-probability group is ~5.0 %, in the ”moderate”-pretest-probability group it is ~17 % and in the ”high”-pretest-probability group it is ~53 %.
The Spanish national consensus document on diagnosis, risk stratification and treatment of patients with pulmonary embolism [6] estimates that the prevalence of PE in the ”low”-pretest-probability group is ~10 %, in the ”moderate”-pretest-probability group it is ~25 % and in the ”high”-pretest-probability group it is >60 %.
The prevalence for the ”DVT unlikely” pretest probability is ~6 % [1] and the prevalence for ”PE unlikely” pretest probability is ~12 % [2].
This means that the NPVs for the ”unlikely”-pretest-probability groups will be quite similar to the NPVs for the corresponding ”low”-pretest-probability groups (prevalence ~5 % and ~10 %, respectively) and they are therefore not addressed separately.
When the estimated prevalence is used for calculating NPVs for our two hypothetical D-dimer assays, we get the NPVs listed in Table I.
If we compare the values with the FDA requirement of NPV ≥ 97 %, we can see that this requirement is only fulfilled for Assay95 for the ”low”- and ”moderate”-pretest-probability groups. For Assay85 it is only fulfilled for the ”low”-pretest-probability groups.
None of the assays have a sufficiently high NPV to be used for rule-out in the ”high”-pretest-probability groups. If they were used for the ”high” -pretest-probability groups the worst case would be using Assay85 for ruling out ”high”-pretest-probability PE patients. In this case 31 % of those dismissed would actually be sick and at high risk of a fatal PE. This is because the NPV is 69 %.
It means that when we dismiss patients with a negative D-dimer result, 69 % of them will not be sick but the remaining 31 % of them WILL be sick.
| Pretest probability | |||||
| Disease state | Assay | Sensitivity* | ”Low” | ”Moderate” | ”High” |
| Negative predictive value, % | |||||
| DVT | Assay95 | 95 % | 100 | 98 | 90 |
| Assay85 | 85 % | 98 | 94 | 75 | |
| PE | Assay95 | 95 % | 99 | 97 | 87 |
| Assay85 | 85 % | 97 | 91 | 69 | |
TABLE I: Negative predictive values for different pretest-probability groups and for DVT and PE
*Specificity = 50 %
The data from Table I is illustrated graphically in Fig. 5 and Fig. 6; see below.
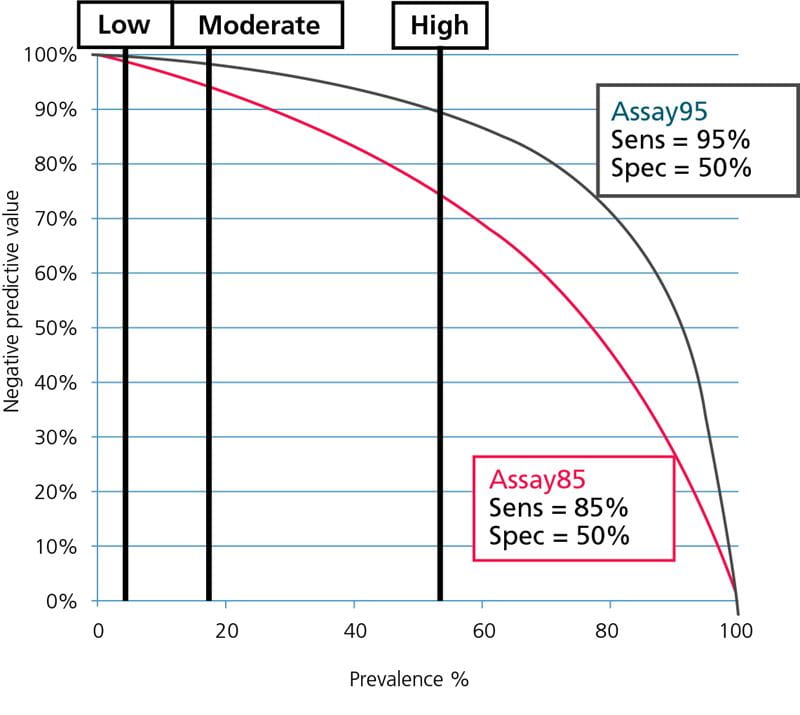
FIG. 5: Negative predictive values as function of prevalence. Prevalence of DVT for ”low”-, ”moderate”- and ”high”- pretest-probability groups shown.
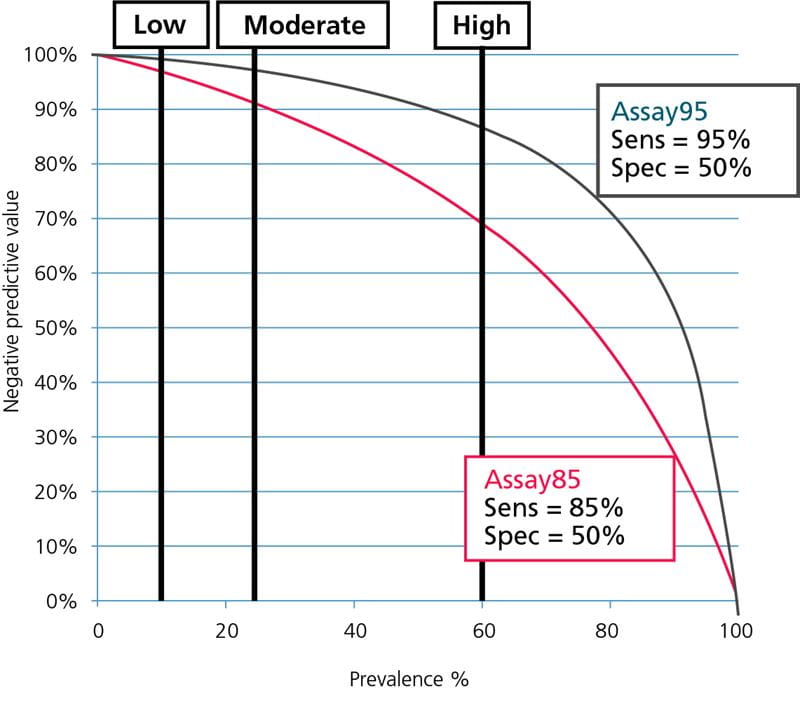
FIG. 6: Negative predictive values as function of prevalence. Prevalence of PE for ”low”-, ”moderate”- and ”high”-pretest-probability groups shown.
From Fig. 5 and Fig. 6 it can be seen that Assay85 with the lowest sensitivity can only be used in populations with a very low prevalence of DVT or PE, corresponding to the prevalence in ”low”-pretest-probability groups, whereas Assay95 with the high sensitivity can be used over a broader prevalence range, corresponding to the prevalence in ”low”- and ”moderate”-pretest-probability groups.
None of the assays can be used in groups with a high prevalence corresponding to the prevalence for ”high”-pretest-probability groups. For the safety of the patients this should always be kept in mind.
References+ View more
- Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, Kearon C, Schunemann HJ, Crowther M, Pauker SG, Makdissi R, Guyatt GH. American College of Chest Physicians: Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 Suppl): e351S-418S
- Authors/Task Force Members, Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Authors/Task Force Members. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014; pii: ehu283. [Epub ahead of print]
- CLSI. Quantitative D-dimer for the Exclusion of Venous Thromboembolic Disease; Approved Guideline. CLSI document H59-A. Wayne PA: Clinical and Laboratory Standards Institute; 2011
- Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood 2009; 113(13): 2878-87
- Sidelmann JJ, Gram J, Larsen A, Overgaard K, Jespersen J: Analytical and clinical validation of a new point-of-care testing system for determination of D-Dimer in human blood. Throm Res 2010; 126(6): 524-30
- Uresandi F, Monreal M, García-Bragado F, Domenech P, Lecumberri R, Escribano P, Zamorano JL, Jiménez S, Ruiz-Artacho P, Lozano F, Romera A, Jiménez Dl, on behalf of the National Consensus on Diagnosis, Risk Stratification and Treatment of Patients With Pulmonary Thromboembolism: National Consensus on the Diagnosis, Risk Stratification and Treatment of Patients with Pulmonary Embolism. Arch Bronconeumol 2013; 49(12): 534-47.
References
- Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, Kearon C, Schunemann HJ, Crowther M, Pauker SG, Makdissi R, Guyatt GH. American College of Chest Physicians: Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 Suppl): e351S-418S
- Authors/Task Force Members, Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Authors/Task Force Members. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014; pii: ehu283. [Epub ahead of print]
- CLSI. Quantitative D-dimer for the Exclusion of Venous Thromboembolic Disease; Approved Guideline. CLSI document H59-A. Wayne PA: Clinical and Laboratory Standards Institute; 2011
- Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood 2009; 113(13): 2878-87
- Sidelmann JJ, Gram J, Larsen A, Overgaard K, Jespersen J: Analytical and clinical validation of a new point-of-care testing system for determination of D-Dimer in human blood. Throm Res 2010; 126(6): 524-30
- Uresandi F, Monreal M, García-Bragado F, Domenech P, Lecumberri R, Escribano P, Zamorano JL, Jiménez S, Ruiz-Artacho P, Lozano F, Romera A, Jiménez Dl, on behalf of the National Consensus on Diagnosis, Risk Stratification and Treatment of Patients With Pulmonary Thromboembolism: National Consensus on the Diagnosis, Risk Stratification and Treatment of Patients with Pulmonary Embolism. Arch Bronconeumol 2013; 49(12): 534-47.
Register for the related webinar
The Benefit of Using a D-dimer Assay with a High Clinical Specificity
Presented by Karin Strandberg, MD, PhD, Assoc. Prof.
Join the webinarAcute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowScientific webinars
Check out the list of webinars
Radiometer and acutecaretesting.org present free educational webinars on topics surrounding acute care testing presented by international experts.
Go to webinars