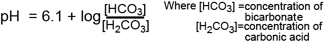Printed from acutecaretesting.org
January 2012
Why measure blood gases? A three-part introduction for the novice - Part 1
SummaryArterial blood gas (ABG) analysis generates a number of parameters (listed in BOX 5) that together allow assessment of pulmonary gas exchange, blood oxygenation and acid-base balance. These physiological functions of the blood, respiratory and renal systems are disturbed in a range of respiratory and non-respiratory diseases.
The application of ABG analysis in diagnosis and monitoring of these diseases will be considered in two future articles, where the focus will be the clinical significance of abnormal ABG results.
RESPIRATORY PHYSIOLOGY
Oxygen is fundamental to life. The cells of all human tissues derive the energy they require to survive and function from the continuous aerobic metabolism of dietary-derived nutrients (carbohydrates, fats, etc.).
This aerobic metabolism requires a constant supply of oxygen and results in a continuous production of carbon dioxide, a waste product that must be eliminated from the body.
The delivery of oxygen to, and removal of carbon dioxide from tissue cells is a major function of blood that occurs in tissues where the smallest blood vessels (microcapillaries) surround each tissue cell.
Blood returning in veins to the heart from this tissue microvasculature (i.e. venous blood) is thus oxygen depleted and relatively rich in carbon dioxide.
Pulmonary gas exchange, central to the process of respiration, accounts for the vital role that the lungs play in the delivery of oxygen to tissue cells and the elimination of carbon dioxide from the body.
The dual objective of respiration is transfer of oxygen present in inspired air to venous blood (blood oxygenation) and elimination of carbon dioxide from venous blood to the environment in expired air.
The site of gaseous exchange between blood and air within the lungs is the alveolar-capillary membrane, which comprises two elements: the alveolar membrane that lines the microscopic, bubble-like, cul-de-sacs of lung structure called alveoli; and the endothelium (wall) of blood microcapillaries.
|
The amount of gas in any system is defined by the pressure it exerts, traditionally measured as height in millimeters (mm) of a column of mercury (Hg). For example, the pressure of atmospheric air (i.e. barometric pressure) at sea level is 760 mmHg. This means that at sea level, the gases contained in the air we breathe have a combined pressure sufficient to support a column of mercury 760 mm high. In a mixture of gases, as air is, the total pressure is simply the sum of the partial pressures (represented by the symbol p) of each gas. Air comprises 21 % oxygen, 0.03 % carbon dioxide and 78% nitrogen so partial pressure of oxygen (pO2) in inspired air is 21 % of total atmospheric pressure (21/100x760) i.e. 150 mmHg and the partial pressure of carbon dioxide pCO2 is 0.03 % of 760, i.e. 0.02 mmHg. The SI unit of pressure used in clinical medicine outside of North America is the kilopascal (kPa). To convert values in the traditional unit (mmHg) to the equivalent SI unit (kPa) value, simply multiply by 0.133. It is important to note that these are a measure only of the amount of gas that is dissolved in arterial blood (or venous blood), not the total amounts. For example, most of the oxygen in blood is bound to the protein hemoglobin. This protein-bound oxygen is not included in the pO2 measurement (see text). |
Maintenance of two measured ABG parameters, pO2(a) and pCO2(a) within normal limits implies effective pulmonary gas exchange, which is dependent on:
- Adequate alveolar ventilation. This is the movement of air in and out of alveoli due to the mechanical process of breathing that depends on the chest musculature and elastic recoil of the lungs.
- Normal numbers of functioning alveoli.
- Normal thickness of alveolar-capillary membrane.
- Sufficient blood flow through pulmonary capillaries (i.e. adequate alveolar perfusion).
- No significant ventilation/perfusion mismatch (mismatch occurs, for example, if alveoli are well perfused with blood but inadequately ventilated with air).
- Intact brain stem (explained below).
Ventilation is continuously regulated, principally by respiratory centers located in the brain stem. These respond to the amount of CO2 in arterial blood (i.e. the pCO2(a)), detected by closely associated chemoreceptor cells. By their neural connection to the chest musculature involved in breathing, these respiratory centers increase the rate and depth of breathing, and thereby increase alveolar ventilation, if pCO2 is rising and reduce alveolar ventilation if pCO2(a) is falling.
By this means sufficient CO2 is eliminated in expired air to maintain pCO2(a) within narrow normal limits.
Inappropriately reduced ventilation (hypoventilation) leads to inadequate pulmonary gas exchange, evident on blood gas analysis as increased pCO2 and decreased pO2(a).
Hyperventilation (overbreathing) is always associated with decreased pCO2(a) but not necessarily, as might be supposed, increased pO2(a).
BLOOD TRANSPORT OF OXYGEN - TWO ABG PARAMETERS (pO2(a) AND sO2)
Partial pressure of oxygen in arterial blood (pO2(a)) is not the only parameter measured during ABG that reflects blood oxygenation; the other is oxygen saturation (sO2).
As is the convention with pO2 (see BOX 1), the symbol for sO2 of specifically arterial blood includes the suffix ”a” so the ABG parameter of oxygen saturation is sO2(a).
To understand the difference and relationship between pO2(a) and sO2(a), we must examine how oxygen is transported in blood.
Oxygen is poorly soluble in blood and the small amount of oxygen that can be transported simply dissolved in blood (~3.0 mL of oxygen per liter of blood) is quite inadequate to satisfy tissue demand for oxygen.
The oxygen-carrying protein, hemoglobin, contained in the cells of blood (specifically the red cells or erythrocytes) provides an additional, far more effective, means of transporting oxygen, and increases the oxygen-carrying capacity of blood from ~3.0 to ~200 mL oxygen per liter.
In fact only 1-2 % of the oxygen transported in blood is dissolved in the aqueous phase of blood; this is the portion that is measured by the pO2(a).
The remaining 98-99 % is transported in erythrocytes bound to hemoglobin.
Each erythrocyte contains 250-300 million hemoglobin molecules and each hemoglobin molecule can bind a maximum of four oxygen molecules.
The product of the reversible binding of oxygen by hemoglobin is called oxyhemoglobin; the term deoxyhemoglobin is used to describe hemoglobin that has no oxygen bound to it.
The oxygen delivery function of hemoglobin, i.e. its ability to ”pick up” oxygen in the lungs and ”release” it in the microvasculature of tissue cells, is made possible by a reversible conformational change in the quaternary structure (shape) of the hemoglobin molecule that alters its affinity for oxygen. In the deoxy state hemoglobin has low affinity for oxygen and in the oxy state it has high affinity for oxygen.
A number of environmental factors in blood determine the hemoglobin state (deoxy or oxy) and thereby the relative affinity for oxygen.
The most significant of these is the pO2. Hemoglobin present in blood with relatively high pO2 has much greater affinity for oxygen than hemoglobin present in blood with relatively low pO2. The oxygen dissociation curve (ODC) describes this relationship graphically (Fig. 1).
The percentage of total hemoglobin saturated with oxygen (i.e. oxygen saturation, sO2) is the measure of hemoglobin affinity in this graph.
It is clear from the graph that at the high pO2 that prevails in the blood exposed to alveolar air in the lung (~13 kPa), hemoglobin is almost 100 % saturated with oxygen; nearly all of the available oxygen-binding sites on the totality of hemoglobin molecules are occupied with oxygen.
By contrast in the milieu of the tissues where pO2 is much lower, hemoglobin affinity for oxygen is also much lower and oxygen is released from hemoglobin to the tissues.
The hemoglobin in venous blood leaving the tissues consequently has less oxygen bound to it and this is reflected in the much lower sO2 of venous blood (sO2(v) ~70 %) compared to that of arterial blood (sO2(a) >95 %).
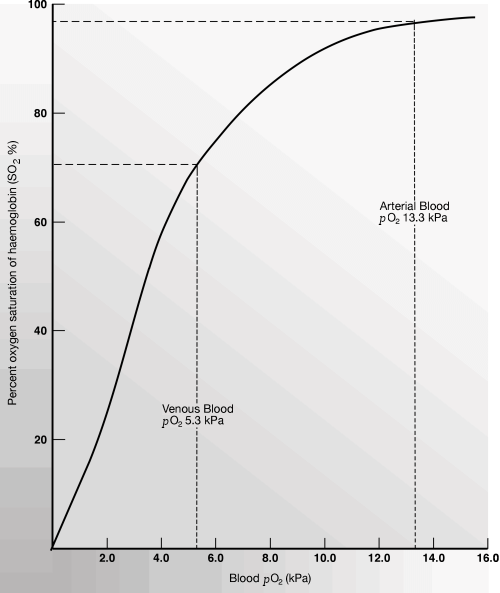
Fig.1: Oxygen dissociation curve. Relationship between the amount of oxygen dissolved in blood (PO2) and the amount of oxygen carried by hemoglobin (SO2).
Although pO2(a) only reflects a tiny proportion (1-2 %) of the oxygen in arterial blood, it is highly significant because it determines the amount of oxygen bound to hemoglobin (the sO2(a)) and thereby the total amount of oxygen that is contained in arterial blood for delivery to tissues. If pO2(a) is reduced, then less oxygen can be carried by hemoglobin (i.e. sO2(a) is reduced) and less oxygen is available to tissues.
Examination of the oxygen dissociation curve (Fig. 1) reveals that a significant decrease in pO2(a) from 16 kPa to 10 kPa has only slight effect on sO2(a) and therefore the oxygen-carrying capacity of blood, but there is a sharp fall in sO2(a) as pO2(a) falls below 10 kPa. The delivery of oxygen to tissues becomes increasingly compromised as pO2(a) falls below this level.
For optimum oxygenation of tissues:
- Blood must contain an adequate amount of hemoglobin.
- That hemoglobin must be >95 % saturated with oxygen in arterial blood (sO2(a) >95 %).
- To achieve sO2 (a) >95 %, pO2 (a) must be >10.6 kPa (80 mmHg).
- Maintenance of pO2(a) above 10.6 kPa depends on the factors required for effective pulmonary gas exchange (see above).
ACID-BASE BALANCE: THE MAINTENANCE OF NORMAL BLOOD pH
In this section we turn the attention away from the ABG parameters that reflect blood oxygenation (pO2(a), sO2(a)) to those that reflect acid-base balance. They are: pH, pCO2(a), bicarbonate concentration (HCO3– ) and base excess. pCO2(a) has already been introduced in the discussion of pulmonary gas exchange, and its inclusion here reflects the central role that the lungs play in the maintenance of blood pH.
All biochemical reactions are sensitive to change in pH, so that optimum survival and function of cells require that blood pH is maintained within the narrow range of 7.35-7.45, despite normal cell metabolism being associated with the production of metabolic acids.
Even mild excursion outside the normal range has multiple deleterious effects, and a pH of less than 6.8 or greater than 7.8 is incompatible with life.
The maintenance of normal blood pH is a complex synergy of action involving the chemical buffers present in blood (principally bicarbonate), red blood cells and the function of three organs: the kidneys, lungs and brain stem.
The following discussion assumes an outline understanding of some basic concepts: pH, acids, bases and buffers (see BOXES 2-4 for a reminder).
|
pH is a logarithmic scale (0 to 14) of acidity/alkalinity. Pure water has a pH of 7 (neutral, i.e. neither acidic nor alkaline). pH above 7is alkaline and pH below 7 is acidic. pH is actually a measure of hydrogen (H) ion concentration and defined as negative log (to the base 10) of the hydrogen ion concentration in moles per litre. thus: From this equation: pH 7.4 = H+ concentration of 40 nmol/L From these examples it is evident that:
Normal arterial blood pH is 7.35 -7.45 (i.e. [H+] of 45-35 nmol/L) |
|
An acid is a substance that dissociates in solution to release hydrogen ions. A base accepts hydrogen ions. For example, hydrochloric acid (HCl) dissociates to hydrogen ions and chloride ions: |
|
A chemical buffer is a compound in solution (the conjugate base of a weak acid) that resists change in the pH of the solution when acid is added, by "mopping up" hydrogen ions. The principal buffer in blood is bicarbonate, which is the conjugate base of the weak acid, carbonic acid. The presence of bicarbonate in blood serves to minimize the change in pH of blood that occurs when acids produced during cell metabolism are released from cells to blood. To illustrate the buffering action of bicarbonate consider a solution of sodium bicarbonate (the buffer) to which a strong acid, in this case hydrochloric acid, is added. The hydrogen ions resulting from strongly dissociating hydrochloric acid are incorporated in to the weak acid, carbonic acid thus: H+Cl- + NaHCO3 ------> H2CO3 + NaCl The pH of any buffered solution is governed by the relative concentration of the weak acid and its conjugate base according to the following so-called Henderson-Hasselbalch equation for bicarbonate buffer in blood: |
To understand the maintenance of blood pH and the other ABG parameters used to assess acid-base balance, it is useful to consider the way carbon dioxide (CO2) is transported in blood from tissue cells to lungs. In the microvasculature of tissues CO2 diffuses from cells –where it is produced – to blood due to the prevailing pCO2 gradient (pCO2 in tissue cells higher than that in blood).
A small amount (~5 %) remains simply dissolved in blood plasma and the cytoplasm of erythrocytes, and a similar amount is carried in erythrocytes bound to hemoglobin that has yielded up its oxygen to tissues, but most (90 %) is hydrated to carbonic acid in erythrocytes by the action of the enzyme carbonic anhydrase. Nearly all (~96 %) of this carbonic acid rapidly dissociates, yielding bicarbonate and hydrogen ions, thus (reaction should be read from left to right):
carbonicanhydrase
CO 2x+ H2O <-----------------> H2CO3 <-----------------> HCO3- + H+
The potentially dangerous fall in red-cell pH induced by the influx of hydrogen ions is ameliorated by them combining with reduced hemoglobin (hemoglobin, now stripped of its oxygen is acting as a buffer here). Around 65% of the bicarbonate passes from erythrocytes and is transported in blood plasma; the rest remains in the cytoplasm of erythrocytes.
When venous blood arrives in the capillary networks that surround the alveoli in the lungs, the small amount of CO2 dissolved in blood passes across the alveolar membrane due to the prevailing pCO2 gradient. This loss of CO2 from blood reverses the direction of the above equation (should now be read from right to left) reflecting a reversal of the sequence of events that occurred in the microvasculature of the tissues. So, in blood perfusing the alveoli, hemoglobin releases hydrogen ions as it combines with inspired oxygen.
These hydrogen ions are buffered by (combine with) bicarbonate to form carbonic acid, which dissociates to carbon dioxide (CO2) and water. The CO2 diffuses from blood to alveoli. The process is continuously regulated so that the amount of CO2 being removed from blood at the lungs equals the amount of CO2 being added to the blood in the tissues.
To summarize, there are four ways in which CO2 is transported in blood:
- 5 % is transported simply dissolved in plasma and erythrocyte cytoplasm. pCO2(a) is a measure of this small portion of total CO2.
- 90 % is transported as bicarbonate (the principal blood buffer) (the concentration of bicarbonate (mmol/L) is calculated during ABG).
- 5 % is transported loosely bound to hemoglobin in red cells.
- <0.1 % is transported as carbonic acid.
THE SYNERGISTIC ROLE OF LUNGS AND KIDNEY IN MAINTAINING NORMAL BLOOD pH
The following relationship between three ABG parameters pH, pCO2(a) and bicarbonate (HCO3–) is derived from the Henderson-Hasselbalch equation for the bicarbonate buffer system in blood (see BOX 4):
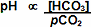
This simple relationship, which is central to an understanding of all essential aspects of acid-base balance and interpretation of patient ABG results, states that pH of arterial blood is a function of both pCO2(a) and bicarbonate concentration. pH falls if either pCO2(a) is increased or bicarbonate is reduced, and pH rises if either pCO2(a) is reduced or bicarbonate is increased. The relationship also reveals that pH remains unchanged if both bicarbonate and pCO2(a) are increased or decreased by the same relative amount.
The inverse relationship between pH and pCO2(a) reflects the acidic potential of carbon dioxide (remember that when dissolved in blood, carbon dioxide is hydrated to carbonic acid, which dissociates yielding hydrogen ions, see equation above). As already discussed, the process of pulmonary gas exchange in the lungs ensures that pCO2(a) remains constant and in so doing ensures a vital contribution to the maintenance of normal pH. Clearly if through disease or injury the lungs were unable to eliminate carbon dioxide efficiently, pCO2(a) would rise and pH would fall.
Bicarbonate concentration, the nominator in the above equation is dependent on normal kidney function.
As the principal buffer in blood, bicarbonate prevents the rapid change in pH that would otherwise occur, when in the normal course of cell metabolism, metabolic acids are released to blood. Bicarbonate ”mops up” the hydrogen ions from these metabolic acids, thereby preventing a fall in pH.
As bicarbonate is consumed in this process, it is of itself a very short-term solution to the problem of maintaining the pH of blood to which acids are being added.
For continuing effectiveness, bicarbonate must be continuously re-generated and the hydrogen ions they are buffering must be removed from the body.
These two tasks are the work of the kidneys. By adjusting the excretion of hydrogen ions and bicarbonate in urine, and thereby the reabsorption of bicarbonate to blood, the kidneys maintain blood concentration of bicarbonate within normal limits. This in turn is essential to the maintenance of normal blood pH.
Maintenance of normal pH depends on:
- Preserving normal pCO2(a) (this in turn depends on adequate pulmonary gas exchange and therefore normally functioning lungs and brain stem).
- Preserving normal bicarbonate concentration (this in turn depends on normally functioning kidneys).
The respiratory and renal systems outlined above operate together in maintaining blood pH. Since preservation of blood pH within the normal range is paramount, the normal response to a fall in bicarbonate is a compensatory adjustment downwards of pCO2(a).
Remember, it is the ratio of bicarbonate: pCO2(a) that must be preserved, if pH is to be maintained within the normal range.
| ABG test | Units | Ref range | Notes |
| Used to access acidbase balance | |||
| pH | pH | 7.35-7.45 | pH is a way of representing hydrogen ion concentration (acidity/alkalinity). |
| Hydrogen ion concentration [H+] |
nmol/L |
35-45 | Alternative to pH reported by some laboratories. |
| pCO2(a) | kPa or mmHg | 4.7-6.0 kPa 35-45 mmHg |
A measure of the amount of CO2 dissolved in blood (only a small fraction (5 %) of total CO2 transported in this way). |
|
Actual bicarbonate (HCO3-) concentration |
nmol/L |
22-28 |
Bicarbonate the principle blood buffermost (90%) of the CO2 in blood transported as bicarbonate. Actual bicarbonate is the actual concentration in the sample - uncorrected for pCO2(a). |
|
Standard bicarbonate (HCO3-) concentration |
nmol/L |
22-28 | Standard bicarbonate is actual bicarbonate corrected for abnormal pCO2(a) concentration (< or > 5.3 kPa) If pCO2(a) is normal then standard bicarb = actual bicarb. The distinction is useful in elucidating acid-base disturbances. |
|
Base excess (BE) |
nmol/L |
-2 to +2 | Base excess is a calulation of the amount of base that needs to be added to or subtrated from blood to acheive a normal pH (7.40) after correction for abnormal pCO2(a) ( |
| Used to access oxygenation status | |||
| pO2(a) | kPa or mmHg |
10.6-13.3 kPa |
A measure of the amount of oxygen simply dissolved in blood. Only a small amount (1-2 %) of the total oxygen in blood is transported in this way. |
| sO2(a) | % | >95% | Oxygen saturation is the % of total hemoglobin oxygen binding sites available in blood that are actually occupied by oxygen. sO2(a) determined by pO2(a). Most (98-99 %) of the oxygen in blood is bound to hemoglobin. |
|
Hemoglobin (Hb) concentration |
g/dL | male 13.5-17.5 female 11.5-15.5 |
Hemoglobin concentration determines the total number of available oxygen-binding sites and therefore the oxygen-carrying capacity of blood. |
References+ View more
- West J. Respiratory physiology - the essentials. 8th ed. Baltimore: Lippincott Williams & Wilkins, 2008.
- Dominiczak M, Szczepanska-Konkel M. Regulation of hydrogen ion concentration (acid-base balance) In: Medical Biochemistry. 3rd ed. Mosby, 2009.
- Waugh A, Grant A. The respiratory system. In: Ross and Wilson anatomy and physiology in health and illness. 11th ed. Churchill-Livingstone, 2010.
- Hennesey I, Japp A. Arterial blood gases made easy. Churchill-Livingstone, 2007.
References
- West J. Respiratory physiology - the essentials. 8th ed. Baltimore: Lippincott Williams & Wilkins, 2008.
- Dominiczak M, Szczepanska-Konkel M. Regulation of hydrogen ion concentration (acid-base balance) In: Medical Biochemistry. 3rd ed. Mosby, 2009.
- Waugh A, Grant A. The respiratory system. In: Ross and Wilson anatomy and physiology in health and illness. 11th ed. Churchill-Livingstone, 2010.
- Hennesey I, Japp A. Arterial blood gases made easy. Churchill-Livingstone, 2007.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar