Printed from acutecaretesting.org
May 2014
Oxygen saturation – better measured than calculated
Oxygen is essential to life. All tissue cells depend for their function and survival on the continuous generation of energy in the form of adenosine triphosphate (ATP); this ATP is generated within cells by aerobic metabolism of dietary fuels (principally glucose) to carbon dioxide and water.
If the supply of oxygen is interrupted, this energy-generating process is curtailed or ceases, with resulting cell injury and, ultimately, cell death and organ failure [1].
Inadequate oxygen within tissues, called hypoxia, is the most common cause of cell injury/death and is central to, or at least a contributory factor in, the etiology and/or pathogenesis of most potentially life-threatening diseases/conditions seen in acute and critical care medicine [2].
To understand how arterial blood gas results help in assessing patient risk of tissue hypoxia, a basic knowledge of oxygen transport in blood is necessary.
OXYGEN TRANSPORT IN BLOOD
A prime function of the respiratory and cardiovascular systems is delivery of inspired (atmospheric) oxygen to tissue cells. This process of delivery begins at the alveolar-capillary membrane of the lungs.
Inspired oxygen present in alveolar air diffuses from alveoli – the microscopic cul-de-sacs of lung structure – to blood flowing through the pulmonary capillaries that surround each alveolus.
Blood, now loaded with oxygen, is conveyed from the lungs via the arterial system to the microvasculature of tissues, where oxygen is released to tissue cells.
Oxygen-depleted blood is conveyed from the tissue microvasculature via the venous system back to the right side of the heart, and onwards via the pulmonary artery to the lungs, for renewed oxygenation.
Oxygen is poorly soluble in blood and the small maximal amount of oxygen that can be transported simply dissolved in blood is quite inadequate to satisfy the body’s demand for oxygen.
In fact, just 1-2 % of the oxygen transported in blood is dissolved in the blood; it is this small fraction that is reflected in the measured partial pressure of oxygen in arterial blood (pO2(a)).
The remaining 98-99 % is transported in erythrocytes bound reversibly to the protein hemoglobin.
The oxygen delivery function of hemoglobin, i.e. its ability to ”pick up” oxygen in the lungs and ”release” it in the microvasculature of tissues is made possible by a reversible change in the structure of the hemoglobin molecule that alters its affinity for oxygen, and thereby the amount of oxygen each molecule carries [3].
A number of environmental factors in blood determine the relative affinity of hemoglobin for oxygen.
The most significant of these is pO2. Hemoglobin present in blood with relatively high pO2 has much greater affinity for oxygen than hemoglobin present in blood with relatively low pO2. The oxygen dissociation curve (ODC) describes this relationship graphically (see Fig. 1).
The percentage of total hemoglobin that is saturated with oxygen (i.e. oxygen saturation, sO2) is the measure of hemoglobin affinity in this graph.
It is clear from the graph that at the high pO2 that prevails in the blood exposed to alveolar air in the lung (~12 kPa), hemoglobin is almost 100 % saturated with oxygen; nearly all of the available oxygen-binding sites on the totality of hemoglobin molecules are occupied with oxygen.
By contrast in the milieu of the tissues where pO2 is much lower, hemoglobin affinity for oxygen is also much lower, and oxygen is released from hemoglobin to the tissues.
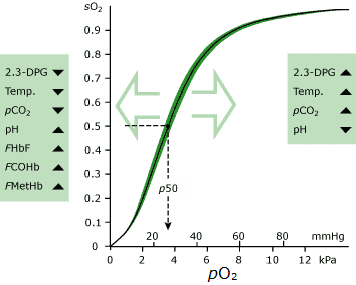
Fig1: OCD
Although pO2(a) only reflects a very small proportion (1-2 %) of the oxygen in arterial blood, it is highly significant because, as the ODC implies, it determines the amount of oxygen bound to hemoglobin in arterial blood (the sO2(a)) and therefore the total amount of oxygen that is contained in arterial blood for delivery to tissues.
If pO2(a) is reduced, then less oxygen can be carried by hemoglobin (i.e. sO2(a) is reduced) and less oxygen is available to tissues. Examination of ODC reveals that a significant decrease in pO2(a) from 15 kPa to 10 kPa has only slight effect on sO2(a) and therefore the oxygen content of arterial blood, but there is a sharp fall in sO2(a) as pO2(a) falls below around 9-10 kPa.
The delivery of oxygen to tissues becomes increasingly compromised as pO2(a) falls below this level.
For adequate oxygenation of tissues:
- blood must contain normal concentration of hemoglobin
- that hemoglobin must be >95 % saturated with oxygen in arterial blood (sO2(a) >95 %)
- to achieve sO2(a) >95 %, pO2(a) must be >10 kPa (see ODC)
- maintenance of normal pO2(a), or at least pO2(a) in excess of 10 kPa, is dependent on an adequate rate of oxygen diffusion from alveoli to pulmonary capillary blood, i.e. normal alveolar ventilation and perfusion
DEFINITION OF ARTERIAL OXYGEN SATURATION (sO2(a))
Oxygen saturation reflects only the oxygen in blood that is bound to hemoglobin, not that tiny amount dissolved in blood plasma.
The hemoglobin molecule is said to be ”saturated” with oxygen when all of its four oxygen-binding sites are occupied with oxygen; the product of this binding is called oxyhemoglobin.
Oxygen saturation is the percentage of total hemoglobin binding sites available for binding to oxygen that is occupied with oxygen.
It is thus a measure of how much of the oxygen-carrying capacity due to hemoglobin is being utilized, and is defined by the following equation:
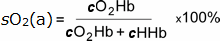 Eqtn 1
Eqtn 1
where cO2Hb = concentration of oxyhemoglobin in arterial blood
cHHb = concentration of deoxyhemoglobin in arterial blood
(cO2Hb + cHHb = concentration of total hemoglobin capable of binding
oxygen)
It is important to note that the denominator in this equation is not the concentration of total hemoglobin.
There are two species of hemoglobin present in blood that are incapable of binding oxygen and are not therefore included in the denominator. They are carboxyhemoglobin (COHb) and methemoglobin (MetHb), together called the dyshemoglobins because of their functional redundancy.
In health, COHb and MetHb together comprise less than ~5 % of total hemoglobin so that, normally, the concentration of total hemoglobin (ctHb) approximates to the sum of cO2Hb and cHHb.
However, there are pathologies – most notably carbon monoxide poisoning and methemoglobinemia – that are associated with a marked increase in COHb or MetHb, and a resulting marked reduction in the oxygen-carrying capacity of blood, that is not reflected in sO2(a).
Similarly, reduction in ctHb (i.e. anemia) also reduces the oxygen-carrying capacity of blood, but elicits no change in sO2(a). Reduction in sO2(a) only arises as a result of conditions (pulmonary and non-pulmonary) that cause reduction in pO2(a).
sO2(a) (or SpO2) within the (normal) reference range (95-98 %) is thus no guarantee that blood is well oxygenated, far less that tissues are adequately oxygenated.
MEASUREMENT OF sO2(a) BY CO-OXIMETRY
Many modern blood gas analyzers have an incorporated CO-oximeter that allows direct measurement of sO2(a). This measurement is based on spectrophotometric analysis of the hemoglobin released from a sample of hemolyzed arterial blood [4].The four hemoglobin species present in blood (oxyhemoglobin, O2Hb; deoxyhemoglobin, HHb; carboxyhemoglobin, COHb; and methemoglobin, MetHb) each have a characteristic light-absorption spectrum.
Measurement of the amount of light absorbed by the hemolyzed sample at multiple specific wavelengths allows accurate determination of the concentration of each of the four hemoglobin species. Concentration of O2Hb and HHb allows sO2(a) to be deduced (see equation 1 above).
This method of sO2(a) measurement allows simultaneous generation of further parameters:
- total hemoglobin, ctHb (cO2Hb + cHHb + cCOHb + cMetHb)
- fractionated carboxyhemoglobin, FCOHb (cCOHb / ctHb × 100)
- fractionated methemoglobin, FMetHb (cMetHb / ctHb × 100)
- fractionated oxyhemoglobin FO2Hb (cO2Hb / ctHb × 100)
CALCULATION OF sO2(a)
Prior to the development of blood gas analyzers with incorporated CO-oximeters,sO2(a) could only be generated during blood gas analysis by calculation from measured pO2(a).
Some blood gas analyzers in use today do not have an incorporated CO-oximeter so that generation of calculated sO2(a) values during blood gas analysis continues.
Calculation of sO2(a) from measured pO2(a) is based on the relationship between the two described by the oxygen dissociation curve (ODC); the calculation is a mathematical description of the curve.
Herein lies the potential deficiency of calculated sO2(a), because the shape and position of the ODC is affected by factors other than pO2(a) and sO2(a). The most significant of these are:
- temperature
- pH
- pCO2
- concentration of 2,3-diphosphoglycerate (2,3-DPG)
- concentration of dyshemoglobins (carboxyhemoglobin, methemoglobin)
The standard (normal) ODC relates pO2(a) and sO2(a) in blood at standard conditions (pH 7.4, pCO2 40 mmHg, and temperature 37 °C). This standard curve also assumes normal concentrations of 2,3-DPG and dyshemoglobin (COHb and MetHb).
The curve is shifted to the right (meaning lower sO2(a) for a given pO2(a)) by any of the following:
- Increased temperature >37 °C
- Increased pCO2 >40 mmHg, 5.3 kPa
- Decreased pH <7.4
- Increased 2,3-DPG
The curve is shifted to the left (meaning higher sO2(a) for a given pO2(a)) by any of the following:
- Decreased temperature <37 °C
- Decreased pCO2 <40 mmHg, 5.3 kPa
- Increased pH >7.4
- Increased concentration of dyshemoglobin (COHb or MetHb)
- Decreased 2,3-DPG
To better understand how these variables affect the ODC, it is useful to view a virtual interactive oxygen dissociation curve; one is available at: www.ventworld.com/resources/oxydisso/dissoc.html
For the generation of calculated sO2(a), blood gas analyzers employ any one of a number of complex algorithms that have been developed for calculation of sO2(a) from measured pO2(a) [5].
In one way or another, they all attempt to take account of some of the variables outlined above that affect the ODC. These algorithms require input not only of measured pO2(a) but also measured pH and in some cases measured pCO2(a) or calculated base excess.
All assume normal 2,3-DPG and some assume no abnormal increase in the dyshemoglobins, COHb and MetHb.
Whilst these algorithms provide sufficiently accurate estimation of sO2(a) for healthy individuals and most patient groups without hypoxemia, this is not necessarily the case for the hypoxemic critically ill patient who may additionally have: severe acid-base disturbance; be hypothermic or hyperthermic; abnormal 2,3-DPG or marked increase in dyshemoglobins [6].
In other words, given the number of factors that affect the ODC as well as the complex interactions between these factors, it is simply not possible with a single mathematical relationship, no matter how sophisticated, to describe sufficiently accurately the precise shape and position of the oxygen dissociation curve for all blood samples from critically ill patients.
The potential inaccuracy associated with calculating sO2(a) from a single mathematical interpolation of the oxygen dissociation curve is well demonstrated by results of a study analysis of 10,079 arterial blood gas results, all derived from patients whose clinical condition required blood gas analysis (i.e. acute or critically sick individuals).
This study [7] revealed that for a pO2(a) value of 8.0 kPa (apparent in 978 of the 10,079 samples) the median calculated sO2(a) was 90.1 %; 90 % of these 978 samples had calculated sO2(a) in the range 87-94 %, but overall the calculated sO2(a) values for 978 patients with a pO2(a) of 8.0 kPa ranged from 67 % (indicating very severe hypoxemia) to 99.4 % (indicating well-oxygenated blood).
Since calculated sO2(a) is based on interpolation of the ODC, errors are inevitably greater for hypoxemic arterial samples and all venous samples, because these are examining the steep part of the curve where quite small errors in pO2 measurement have marked effect on sO2.
CALCULATED (ESTIMATED) sO2(a) AND sO2(v) SHOULD NOT BE USED TO CALCULATE OTHER VARIABLES OF OXYGEN TRANSPORT, DELIVERY AND CONSUMPTION
Oxygen saturation values in arterial bloodsO2(a) and mixed venous blood (sO2(v)) are used in calculations to determine other clinically useful parameters for assessment of hypoxia risk among the critically ill [8].
The major reason for preference of directly measured oxygen saturation over calculated (estimated) oxygen saturation is based on the notion that the inherent potential error in calculating oxygen saturation outlined above is amplified during calculation of these additional parameters.
To understand how this error amplification may occur, it is important first to define some of these derived parameters: ctO2(a), DO2, and VO2.
Full assessment of oxygen delivery to tissue requires knowledge of the total oxygen content of arterial blood, ctO2(a). This is the sum of the oxygen dissolved in blood and the oxygen bound to hemoglobin [9] and is calculated during arterial blood gas analysis using the following equation:
ctO2(a) (ml/L) = (k1 × ctHb x sO2(a)) + (k2 × pO2(a)) Eqtn 2
where ctHb = concentration of total hemoglobin (g/L)
sO2(a)= oxygen saturation of arterial blood (%)
pO2(a) = partial pressure of oxygen in arterial blood (kPa)
k1 is a constant (the oxygen-binding capacity of hemoglobin) = 1.31 mL/g
k2 is a constant (solubility coefficient of oxygen at 37 °C) = 0.23
mL/L/kPa)
ctO2(a), in turn, allows calculation of global oxygen delivery (DO2), i.e. the volume of oxygen delivered from lungs to tissues every minute [9]. This is dependent on two parameters: concentration of oxygen in arterial blood and total blood flow in unit time (i.e. cardiac output, CO) and is expressed by the following equation:
DO2 (mL/min) = ctO2(a) × CO Eqtn 3
where CO = cardiac output in mL/min (normally around 5 L/min)
This relationship highlights the fact that tissue hypoxia can (and often does) occur despite normal blood oxygenation. Adequate delivery of oxygen to tissues is threatened not only by inadequate blood oxygenation but also by reduced blood flow.
Knowledge of ctO2(a) also allows calculation of global oxygen consumption (VO2), i.e. the volume of oxygen consumed by tissues in unit time [9]. This calculation also requires knowledge of ctO2(v), the concentration of oxygen in mixed venous blood.
This is generated during blood gas analysis of blood sampled via a pulmonary artery catheter (i.e. mixed venous blood) [10]. It is calculated from measured partial pressure (pO2(v)), oxygen saturation (sO2(v)) and hemoglobin concentration (ctHb) as in equation 2 (above) for arterial blood.
The equation for the calculation of VO2 is:
VO2 (mL/min) = CO × [ctO2(a) – ctO2(v)] Eqtn 4
The risk of tissue hypoxia is increased if tissues are consuming supranormal amounts of oxygen (i.e. VO2 is increased), as might well be the case for some patients suffering critical illness [11].
Clearly, the accuracy of all these derived parameters depends in large part on the accuracy of oxygen saturation values (sO2(a) and sO2(v)).
A number of studies [12, 13, 14, 15] have demonstrated a clinically significant discrepancy if calculated values for sO2(a)/sO2(v), rather than CO-oximeter-generated measured values, are used to determine these derived parameters.
The authors of all these studies conclude that for clinically reliable estimation of derived variables such as VO2 and DO2, sO2(a) and sO2(v) must be measured directly by CO-oximetry; calculated values are not suitable.
This same advice is contained in guidelines from the Clinical and Laboratory Standards Institute [16].
SUMMARY
- Oxygen saturation (sO2) is a parameter used in clinical medicine to assess blood oxygenation and by extension, risk of tissue hypoxia.
- Oxygen saturation is most commonly monitored non-invasively by pulse oximetry, but this approach has limitations.
- A fuller and more accurate assessment of blood oxygenation is offered by arterial blood gas analysis. Oxygen saturation is just one of several oxygen-related parameters generated during blood gas analysis.
- Oxygen saturation is generated during blood gas analysis by one of two methods: direct measurement by CO-oximetry; or calculated from measured pO2.
- The calculation used to generate sO2 from pO2(a) is based on the relationship between the two described by the oxygen dissociation curve.
- The oxygen dissociation curve is affected by a number of factors other than pO2 and sO2 that may be in a state of considerable flux during critical illness, rendering calculated sO2 potentially inaccurate.
- Measured sO2 (by CO-oximetry) is unaffected by these fluxes; it is the method of choice for determining oxygen saturation and the most commonly used nowadays (most modern blood gas analyzers have an incorporated CO-oximeter)
- Clinicians should be aware of the method used to generate sO2 during blood gas analysis at their institution. If the method is calculation from measured pO2, then sO2 values from critically ill patients should be interpreted with caution. Discrepancy between pO2(a) and calculated sO2 (for example, one indicating hypoxemia and the other indicating normoxemia) suggests an inaccurate calculated sO2(a) value.
- Calculated sO2 values should not be used to calculate further oxygen-related variables such as DO2 and VO2; only directly measured sO2 values should be used in these calculations.
References+ View more
- Gutierrez J, Theidorou A. Oxygen delivery and oxygen consumption in Pediatric Critical Care. In: Lucking S, Maffei F et al, eds. Pediatric Critical Care Study Guide. London: Springer 2012, chapter 2.
- Hameed S, Aird W, Cohn S. Oxygen delivery. Crit Care Med 2003; 31, 12: 658-67.
- Ranney H, Aharma V. Structure and function of hemoglobin, In: Beutler E, Lichtman M et al, eds. Williams’s Hematology. 6th edition, 2000.
- Zijlstra W, Bursma A, Zwart A. Performance of an automated six wavelength photometer (Radiometer OSM3) for routine measurement of hemoglobin derivatives. Clin Chem 1988; 34: 149-52
- Breuer HW, Groeben H, Breuer J, Worth H. Oxygen saturation calculation procedures: a critical analysis of six equations for the determination of oxygen saturation. Intensive Care Medicine 1989; 15: 385-89.
- Morgan T. The oxyhaemoglobin dissociation curve in critical illness. Critical Care and Resuscitation 1999; 1: 93-100
- Gothgen I, Siggard-Anderson O, Kokholm G: Variations in the hemoglobin dissociation curve in 10079 arterial blood samples. Scand J Clin Lab Invest 1990; 50, 203: 87-90.
- Johnson K. Diagnostic measures to evaluate oxygenation in critically ill adults. AACN 2004; 15: 506-24
- McLellan S, Walsh T. Oxygen delivery and haemoglobin. Continuing Education in Anaesthesia, Critical Care and Pain 2004; 4: 123-26.
- Zaja J. Venous oximetry. Signa Vitae 2007; 2: 6-10
- Villar J, Slutsky A, Hew E et al. Oxygen transport and oxygen consumption in critically ill patients. Chest 1990; 98: 687-92
- Hess D, Elser R, Agarwal N. The effects on the pulmonary shunt value of using measured versus calculated saturation and of correcting for the presence of carboxyhemoglobin and methemoglobin. Respiratory Care 1984; 29: 1001-05.
- Johnson P, Bihari D, Raper R et al. A comparison between direct and calculated oxygen saturation in intensive care. Anaesth Intensive Care 1993; 21: 72-75.
- Mybergh J. Derived oxygen saturations are not clinically useful for the calculation of oxygen consumption. Anaesth Intensive Care 1992; 20: 460-463.
- Woda R, Dzwonczyk R, Orlowski J et al. Effect of measurement error on calculated variables of oxygen transport. J Applied Physiol 1996; 80: 559-63.
- Clinical and Laboratory Standards Institute. Blood gas and pH analysis and related measurements: Approved guidelines, National Committee for Clinical Laboratory Standards. C46-A2, 2009, 29.
References
- Gutierrez J, Theidorou A. Oxygen delivery and oxygen consumption in Pediatric Critical Care. In: Lucking S, Maffei F et al, eds. Pediatric Critical Care Study Guide. London: Springer 2012, chapter 2.
- Hameed S, Aird W, Cohn S. Oxygen delivery. Crit Care Med 2003; 31, 12: 658-67.
- Ranney H, Aharma V. Structure and function of hemoglobin, In: Beutler E, Lichtman M et al, eds. Williams’s Hematology. 6th edition, 2000.
- Zijlstra W, Bursma A, Zwart A. Performance of an automated six wavelength photometer (Radiometer OSM3) for routine measurement of hemoglobin derivatives. Clin Chem 1988; 34: 149-52
- Breuer HW, Groeben H, Breuer J, Worth H. Oxygen saturation calculation procedures: a critical analysis of six equations for the determination of oxygen saturation. Intensive Care Medicine 1989; 15: 385-89.
- Morgan T. The oxyhaemoglobin dissociation curve in critical illness. Critical Care and Resuscitation 1999; 1: 93-100
- Gothgen I, Siggard-Anderson O, Kokholm G: Variations in the hemoglobin dissociation curve in 10079 arterial blood samples. Scand J Clin Lab Invest 1990; 50, 203: 87-90.
- Johnson K. Diagnostic measures to evaluate oxygenation in critically ill adults. AACN 2004; 15: 506-24
- McLellan S, Walsh T. Oxygen delivery and haemoglobin. Continuing Education in Anaesthesia, Critical Care and Pain 2004; 4: 123-26.
- Zaja J. Venous oximetry. Signa Vitae 2007; 2: 6-10
- Villar J, Slutsky A, Hew E et al. Oxygen transport and oxygen consumption in critically ill patients. Chest 1990; 98: 687-92
- Hess D, Elser R, Agarwal N. The effects on the pulmonary shunt value of using measured versus calculated saturation and of correcting for the presence of carboxyhemoglobin and methemoglobin. Respiratory Care 1984; 29: 1001-05.
- Johnson P, Bihari D, Raper R et al. A comparison between direct and calculated oxygen saturation in intensive care. Anaesth Intensive Care 1993; 21: 72-75.
- Mybergh J. Derived oxygen saturations are not clinically useful for the calculation of oxygen consumption. Anaesth Intensive Care 1992; 20: 460-463.
- Woda R, Dzwonczyk R, Orlowski J et al. Effect of measurement error on calculated variables of oxygen transport. J Applied Physiol 1996; 80: 559-63.
- Clinical and Laboratory Standards Institute. Blood gas and pH analysis and related measurements: Approved guidelines, National Committee for Clinical Laboratory Standards. C46-A2, 2009, 29.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









