Printed from acutecaretesting.org
July 2014
Postmortem CO-oximetry
HEMOGLOBIN AND ITS DERIVATIVES
Hemoglobin (Hb) is the oxygen-carrying protein contained in red blood cells (erythrocytes) that ensures constant delivery of oxygen present in inspired air from the lungs to all tissue cells; each red blood cell contains approximately 250-300 million Hb molecules.
The hemoglobin molecule comprises four folded chains of amino-acids (the globin portion of the molecule). Each of these four globin units has a much smaller heme group attached, and at the center of each heme group is an atom of iron in the ferrous state (Fe2+) [1].
The four atoms of (heme) iron represent the functional center of the hemoglobin molecule because these are the sites for reversible binding of oxygen. Each hemoglobin molecule thus has the capacity to reversibly bind a maximum of four molecules of oxygen.
The product of oxygen binding to hemoglobin is called oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb) is hemoglobin without bound oxygen.
In health, the hemoglobin in blood leaving the lungs and throughout the arterial system is close to 100 % saturated with oxygen; i.e. around 95-98 % is present as O2Hb and around 2-5 % is present as HHb.
The hemoglobin in blood leaving the capillary tissue bed (after oxygen delivery) and throughout the venous system is, by contrast, significantly less saturated with oxygen, i.e. around 70 % is present as O2Hb and around 30 % is present as HHb [1].
Apart from O2Hb and HHb, blood normally contains trace amounts of two further hemoglobin species – carboxyhemoglobin (COHb) and methemoglobin (MetHb).
These two species are incapable of binding oxygen and are consequently collectively referred to as the dyshemoglobins. COHb is the result of hemoglobin binding carbon monoxide rather than oxygen at the heme iron sites [2].
MetHb differs from other hemoglobin species in one important respect: one or more of the four heme iron atoms is in the oxidized ferric (Fe3+) state rather than the reduced ferrous (Fe2+) state [3].
This renders MetHb incapable of binding oxygen. In health, COHb and MetHb together comprise less than 2-3 % of total hemoglobin.
Increase in dyshemoglobin (either COHb or MetHb), a condition called dyshemoglobinemia, reduces the oxygen-carrying capacity of blood and thereby increases the risk of tissue hypoxia; severe increase is potentially lethal.
The principal cause of increased COHb is breathing air that is unnaturally polluted with carbon monoxide [2].
The most common cause of increased MetHb is exposure to oxidizing chemicals, including a range of prescribed and self-prescribed drugs [3].
Finally for completeness, mention must be made of sulfhemoglobin (SulfHb). This, in common with COHb and MetHb, is a hemoglobin species that is incapable of carrying oxygen but, unlike these other two dyshemoglobins, is normally undetectable in the blood of healthy individuals.
SulfHb is formed by incorporation of a sulphur atom in the heme portion of the hemoglobin molecule and is a very rare cause of dyshemoglobinemia that can occur after exposure to sulphur-containing drugs/chemicals or pathological accumulation of hydrogen sulphide (H2S) in tissues [4, 5].
PRINCIPLES OF CO-OXIMETRY AND UTILITY OF CO-OXIMETRY POSTMORTEM
Spectrophotometric analysis of blood is the basis of CO-oximetry.
Spectrophotometric analysis allows quantitative measurement of a substance in a solution by measuring the difference in intensity of incident and transmitted light when the solution is placed in the path of a light source of defined wavelength; the difference is called the absorbed light or absorbance.
Application of this general technique in measurement of hemoglobin derivatives (O2Hb, HHb, COHb, MetHb and SulfHb) by CO-oximetry is based on the fact that each of the hemoglobin derivatives has a unique absorbance spectrum [6].
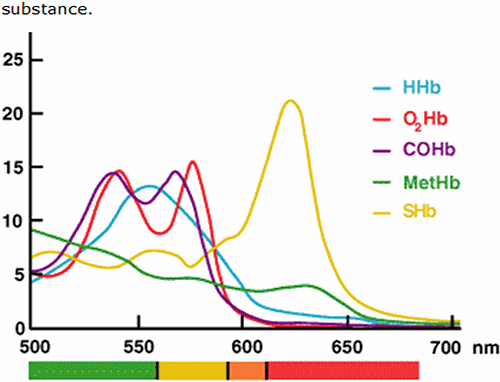
FIG. 1: Absorbance spectrum of hemoglobin derivatives.
That is to say the maximum absorbance of each differs according to the wavelength of incident light. Beer-Lambert’s law – which underpins spectrophotometric analysis – dictates that absorbance of a single absorbing species in a solution is proportional to the concentration of that compound.
For a solution containing more than one absorbing substance (e.g. a mixture of hemoglobin derivatives), so long as the spectral characteristic of each absorbing substance is known, absorbance readings of the solution at multiple wavelengths can be used to compute the concentration of each absorbing substance [6, 7, 17].
A well-mixed anticoagulated arterial or venous blood sample is simply injected into the blood gas analyzer/CO-oximeter.
The sample, or a measured portion of it, is automatically pumped to the temperature-controlled measuring cuvette of the CO-oximeter that is situated in the light path, where by either chemical or physical action, erythrocytes are hemolyzed to release hemoglobin which is spectroscopically scanned as outlined above.
Computational algorithm contained within the CO-oximeter allows immediate calculation of each hemoglobin-variant concentration from absorbance readings, and results are automatically displayed in less than a minute as % of total hemoglobin for the four hemoglobin derivatives (plus SulfHb, if present), and as concentration (g/dL or g/L) for total hemoglobin (ctHb).
Although all CO-oximeter-generated parameters (ctHb, O2Hb, HHb, COHb, MetHb) are clinically useful, only one of these, COHb has really proven useful postmortem. The lack of value in measuring O2Hb and HHb postmortem was confirmed in a study of 214 forensic autopsy cases [8].
O2Hb in left- and right-heart blood ranged from 0 to 97 %. No correlation was found between % O2Hb and the following: postmortem interval (time since death); rectal temperature; and cause of death.
A similar study [9] focused on the value of measuring MetHb postmortem. Blood was sampled from the heart at 49 unselected autopsies; postmortem interval ranged from 2 to 72 hours (mean 24.67 hours).
CO-oximetric analysis revealed that MetHb ranged from 0 to 57 % (mean 17.3 %). Although this study confirmed that increased methemoglobin production often occurs during the process of postmortem autolysis, the actual level provides no useful information.
There was no correlation between % MetHb and any of the following: antemortem circumstances of death; autopsy findings; and postmortem interval.
Crucially, postmortem MetHb measurement is not helpful in confirming or excluding methemoglobinemia (increased MetHb) as a cause of death.
The utility of CO-oximetric analysis of blood samples after death is thus confined to measurement of COHb in order to help confirm that carbon monoxide poisoning contributed to death.
The most common context for such deaths is fires [10] and intentional (suicidal) exposure to motor-vehicle exhaust [11], but there are many others including: aircraft accidents [12], exposure to carbon monoxide in wood stores [13] and – in a domestic setting – inadequately maintained domestic heating appliances in poorly ventilated properties.
CARBON MONOXIDE POISONING AND THE ROLE OF POSTMORTEM COHb MEASUREMENT
The trace amount of COHb (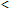 1-2 % of ctHb) normally present in blood is due to endogenously produced carbon monoxide during catabolism of heme [14].
1-2 % of ctHb) normally present in blood is due to endogenously produced carbon monoxide during catabolism of heme [14].
Although the pathological increased catabolism of heme associated with hemolytic disease can result in increased endogenous production of carbon monoxide and resulting increase in COHb, the effect is slight and generally associated with an increase in COHb of only 2-3 %, which is insufficient to have any symptomatic or clinical effect.
Practically, the only cause of clinically significant increase in COHb is breathing air that is unnaturally polluted with carbon monoxide, i.e. carbon monoxide poisoning [15].
The leading artificial sources of carbon monoxide in environmental air are exhaust from motor vehicles, lawn mowers etc. and incomplete combustion of any carbon-containing fuel (wood, charcoal, oil, propane, natural gas etc.) [16].
Cigarette smoke contains carbon monoxide, so that COHb is higher (5-13 %) among regular smokers compared with non-smokers (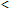 2 %) [16].
2 %) [16].
Carbon monoxide intoxication is the leading cause of unintentional poisoning in Western countries, accounting for 15,000 emergency room admissions and 500 deaths each year in the US [16].
In addition, deliberate exposure to carbon monoxide (e.g. vehicle exhaust) in an enclosed space is a method employed by those with suicidal intent.
The toxicity of carbon monoxide is due to its ability to bind hemoglobin to form COHb. Affinity of hemoglobin for carbon monoxide is 200-250 greater than that for oxygen.
Not only does carbon monoxide displace oxygen from hemoglobin but it alters the allosteric structure of hemoglobin such that the affinity of the remaining sites for bound oxygen increases, effectively decreasing release of oxygen to tissues.
Thus carbon monoxide reduces the oxygen-carrying capacity of blood and reduces delivery of oxygen to tissues. Organs with the highest oxygen demand such as the brain and heart are most vulnerable to the resulting potential tissue hypoxia, which first becomes symptomatically evident as COHb rises above 10 % [17].
Mild to moderately severe carbon monoxide intoxication (COHb in the approximate range of 10-30 %) is associated with any of the following non-specific symptoms: headache, fatigue, nausea, vomiting, dizziness.
Severe intoxication (COHb 30-50 %) can cause coma, convulsions and depressed respiratory status. Most cases of fatal carbon monoxide poisoning are associated with COHb in excess of 50 % [18].
Those with pre-existing chronic respiratory or cardiovascular disease may be predisposed to hypoxia; these patients are thus more vulnerable to carbon monoxide poisoning and death may occur even if COHb is significantly less than 50 %.
METHODS USED TO MEASURE COHb POSTMORTEM – IS CO-OXIMETRY RELIABLE?
Three broad analytical methodologies are available for the postmortem measurement of COHb [18]. They are:
- Gas chromatography
- Manual (two-wavelength) spectrophotometry
- Automated spectrophotometry (CO-oximetry)
Gas chromatography methods, which in essence are all based on measurement of the carbon monoxide liberated from a treated blood sample, are the most sensitive, precise and accurate, and widely considered to be the reference methods of choice [12, 18].
They are, however, expensive, technically demanding, time-consuming and require separate measurement of ctHb [18]. For these reasons the use of gas chromatography for measurement of COHb tends to be confined to well-resourced research centers.
Manual spectrophotometer methods require that absorbance measurements at two wavelengths are made before and after blood is treated with a reducing agent that converts both O2Hb and MetHb to HHb. Importantly, COHb is unaffected by this treatment.
The two measuring wavelengths distinguish COHb and HHb. Prior to the development of CO-oximeters from the early 1980s, these manual methods were widely used.
Because of their relative simplicity, and the fact that the spectrophotometer is a common laboratory instrument with application beyond measurement of COHb, these methods continue to be used in a minority of laboratories [18].
CO-oximetry is currently the most widely used method of measuring COHb postmortem principally due to its ease and speed of operation (analysis time 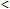 1 minute) [19], but it must be remembered that CO-oximeters were developed for clinical use and the analysis of fresh blood.
1 minute) [19], but it must be remembered that CO-oximeters were developed for clinical use and the analysis of fresh blood.
Their use for analyzing blood and cavity fluid samples recovered after death raises some issues of reliability [17-20]. Three features of significance for measurement of COHb by CO-oximetry distinguish fresh blood sampled during life from blood collected after death. They are:
- Blood sampled postmortem MAY have significantly higher MetHb [9] and/or SulfHb [20]
- Blood sampled postmortem MAY have significantly lower ctHb [22]
- Blood sampled postmortem MAY not be homogeneous and contain microcoagulates and other particulate matter that is not present in fresh blood sampled during life [19]
These changes can be due to the circumstances of death (e.g. the victims of fire frequently have increased MetHb – an effect of heat [22]) or simply reflect the process of decomposition/putrefaction following death (hemoglobin is denatured, hydrogen sulphide and oxidizing species produced during putrefaction promote production of SulfHb and MetHb, respectively).
MetHb, SulfHb and the turbidity associated with putrefied blood are all potential interferents in the CO-oximetric measurement of COHb. Furthermore, accuracy of CO-oximetry is potentially reduced if total hemoglobin is significantly below that which is present during life.
Manufacturers of CO-oximeters generally advise that the accuracy and precision of hemoglobin-variant measurement is unacceptable if ctHb is 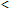 3-4 g/dL (
3-4 g/dL (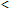 30-40 g/L) [22, 25].
30-40 g/L) [22, 25].
Importantly, the ability of CO-oximeters to correct for interfering species (MetHb, SulfHb, turbidity due to particulate matter) varies, so that some CO-oximeters are more suited to measurement of COHb in postmortem blood than others.
First-generation CO-oximeters make measurements at only four wavelengths but later instruments measure at six or more wavelengths. These more sophisticated CO-oximeters are better equipped to correct for interfering species [10, 23].
One solution to the problem of possible MetHb interference in COHb measurement is to pretreat the sample with a reducing agent (e.g. sodium dithionite) that converts MetHb to HHb [22, 23].
Some authorities recommend filtration of samples prior to measurement to remove debris that could cause interfering turbidity [24].
Despite the potential for interference, the results of study comparing six-wavelength CO-oximetry with a reference gas chromatography method suggest that CO-oximetry can be a reliable method of measuring COHb postmortem even if the sample is putrefied, so long as the ctHb of the sample is 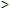 1 g/dL (10 g/L) [25].
1 g/dL (10 g/L) [25].
STABILITY OF COHb WHEN MEASURED BY CO-OXIMETRY
Forensic examination of deaths in which carbon monoxide poisoning are implicated requires that blood samples for COHb estimation be stored in case immediate autopsy is not possible, or re-examination is necessary.
A number of studies [23, 27, 28] have addressed the issue of stability of COHb as measured by CO-oximetry in stored blood samples. They have confirmed that COHb is remarkably stable.
No change in CO-oximetrically determined COHb from baseline was noted in postmortem samples collected into heparin and stored for up to 3 years in a refrigerator [28].
Hampson [27] determined that there was no significant change from baseline in COHb for unrefrigerated samples mailed across the US and back again. And Kunsman et al [23] confirmed that stability of CO-oximetrically determined COHb was not dependant on the use of a particular anticoagulant/preservative combination.
TWO ILLUSTRATIVE CASE HISTORIES
Case history 1
This case [10] exemplifies the use of postmortem CO-oximetry measurement of COHb in forensic homicide investigation. A 46-year-old woman was found dead at home following a fire.
The woman’s husband had started the fire after an argument that resulted in the woman being ”accidentally” knocked unconscious; he apparently believed his wife was dead and admitted starting the house fire in order to cover up the death.
The prosecution case that the husband was legally responsible for the death of his wife hinged on demonstrating that she was alive when the fire was started, and had died from asphyxiation due to smoke (carbon monoxide) inhalation rather than the ”accidental” trauma sustained during the couple’s argument.
Postmortem findings presented by the medical examiner were crucial to this prosecution case. At postmortem examination, blood was sampled for COHb estimation by CO-oximetry.
The result, COHb 61 %, is consistent with the notion that the woman was still breathing (ventilating) during the fire and that the cause of her death was asphyxiation due to carbon monoxide poisoning, consequent on smoke inhalation.
If the wife had been dead before the fire, she could not have inspired carbon monoxide and her postmortem COHb would have been close to normal limits (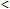 10 % for smokers;
10 % for smokers;  2 % for non-smokers).
2 % for non-smokers).
The postmortem finding of soot particles deep within the woman’s respiratory system (present in both trachea and bronchi) provided further evidence of continued ventilation by the woman during the fire.
During pretrial, defense lawyers argued that CO-oximetry was, compared with other methods, unreliable for postmortem COHb measurement, and that this part of the prosecution case was inadmissible in court.
A clinical chemist provided evidence to the effect that so long as modern multiple-wavelength CO-oximeters (i.e. measuring at six wavelengths or more) are employed, the effect of postmortem-related interferents can be eliminated, and in such circumstance CO-oximetry is a sufficiently reliable technique.
In the light of this expert testimony, the CO-oximetry evidence was judged admissible and the defendant was found guilty of murder by his act of arson.
In commentary of this case study report [26] the author implies that he is not entirely in agreement with the expert testimony presented at pretrial and suggests that the finding of raised COHb in legal cases should always be confirmed by testing using a second method based on a different chemical principle.
Case History 2
This atypical case [11] is notable in that it exemplifies the truth that fatal exposure to vehicle exhaust is not always associated with COHb  50 %; rarely, it can be considerably lower.
50 %; rarely, it can be considerably lower.
The case also highlights one of the contentious issues surrounding the use of CO-oximetry postmortem, namely the potential for MetHb interference in COHb measurement.
A 28-year-old man was found dead in his car many hours after going missing. Suicidal intent was assumed by the finding of a garden hose taped to the exhaust pipe that had delivered exhaust fumes into the cab of the closed vehicle.
At autopsy 5.5 days later blood was sampled for COHb measurement by CO-oximetry to confirm carbon monoxide poisoning. The CO-oximeter used (IL 682) measures at six wavelengths.
Results of this initial testing revealed that COHb was, surprisingly, not increased (0.6 %), but MetHb was severely increased (56.3 %). O2Hb and HHb were 12.2 % and 30.9 % respectively, and total hemoglobin was within normal healthy limits (13.8 g/dL).
It is routine practice at the laboratory where this analysis was done to repeat COHb measurement in cases of potential carbon monoxide poisoning – using a different technique – if CO-oximetry reveals MetHb  30 %.
30 %.
This practice is based on the assumption that increased levels of MetHb can cause falsely low COHb CO-oximetry values.
On confirmatory testing using a reference gas chromatography assay, it was established that the “true” COHb value was 18.6 %, much higher than the CO-oximetry result – confirming carbon monoxide intoxication – but not sufficiently high to conclude that carbon monoxide poisoning was the sole cause of death.
After further laboratory testing, which included confirming the high MetHb by a spectrophotometric assay, the authors of this report concluded that death was due to hypoxia consequent on a combination of carbon monoxide intoxication and acquired severe methemoglobinemia.
In support of this conclusion the authors postulate that the mechanism of severe methemoglobinemia in this case was inhalation of nitrogen oxides, known to be present in exhaust fumes.
References+ View more
- Ranney HM, Sharma V. Structure and function of hemoglobin. In: Beutler E, Lichtman M, eds. Williams Hematology. New York City: McGraw Hill, 2000: 345-53.
- Higgins C. Causes and clinical significance of increased carboxyhemoglobin. www.acutecaretesting.org Oct 2005.
- Higgins C. Methemoglobin. www.acutecaretesting.org Oct 2006.
- Curry S. Hematologic syndromes: hemolysis, methemoglobinemia and sulphhemoglobinemia. In: Brent J, Wallace K, Burkhat K, eds. Critical care toxicology diagnosis and management of the critically poisoned patient. St. Louis: Mosby, 2004.
- Tanggerman A, Bongaerts G, Agbeko R, Semmekrot B, Severijnen R. The origin of hydrogen sulphide in the newborn with sulfhaemoglobin induced cyanosis. J Clin Pathol 2002; 55: 631-33.
- Mahoney J, Vreman H, Stevnson D, Van Kessel A. Measurement of carboxyhemoglobin and total hemoglobin by five specialized spectrohoptometers (CO-oximeters) in comparison with reference methods. Clin Chem 1993; 39: 1693-700.
- Brunelle J, Degtiarov R, Moran R, Race L. Simultaneous measurement of total hemoglobin and its derivatives in blood using CO-oximeters: analytical principles; their application in selecting analytical wavelenghts and reference methods; a comparison of the results of choices made. Scand J Clin Lab Med 1996; 224: 47-69.
- Hitoshu M, Fukita K, Oritani S. Evaluation of postmortem oxymetry with reference to the causes of death. Forensic Sci Int 1997; 87: 201-10.
- Reay D, Insalaco S, Eisele J. Postmortem methemoglobin concentrations and their significance. J Forensic Sci 1984; 29: 1160-63.
- Olsen K, Hillyer M, Kloss J, Geiselhart R, Apple F. Accident or arson: is CO-oximetry reliable for carboxyhemoglobin measurement postmortem? Clin Chem 2010; 56: 515-20.
- Vevelstad M, Morild I. Lethal methemoglobinemia and automobile exhaust inhalation. Forensic Sci Int 2009; 187: 1-5.
- Lewis R, Johnson R, Canfield D. An accurate method for the determination of carbon in postmortem blood using GC-TCD. J Ana Toxicol 2004; 28: 59-62.
- Gauthier S, Grass H, Lory M, Krämer T, Thali M, Bartsch C. Lethal carbon monoxide poisoning in wood pellet storerooms – two cases and a review of the literature. Ann Occup Hyg 2002; 56: 755-63.
- Coburn R, Williams W, Forster RE. Effect of erythrocyte destruction on carbon monoxide production in man. J Clin Invest 1964; 43: 1098-103.
- Ernst A, Zibrak J. Carbon monoxide poisoning. New Eng J Med 1998; 339: 1603-08.
- Lippi G, Rastelli G, Meschi T, Borghi L, Cervellin G. Pathophysiology clinics, diagnosis and treatment of heart involvement in carbon monoxide poisoning. Clin Biochem 2012; 45: 1278-85.
- Widdop B. Analysis of carbon monoxide. Ann Clin Biochem 2002; 39: 378-91.
- Boumba V, Vougiouklakis T. Evaluation of methods used for carboxyhemoglobin analysis in postmortem blood. Int J Toxicol 2005; 24: 275-81.
- Lee CW, Yim LK, Chan DT, Tam JC. Sample pre-treatment for CO-oximetric determination of carboxyhaemoglobin in putrefied blood and cavity fluid. Forensic Sci Int 2002; 126: 162-66.
- Richardson T. Pitfalls in forensic toxicology. Ann Clin Biochem 2000; 37: 20-44.
- Rai V, Minty P. The determination of carboxyhaemoglobin in the presence of sulphaemoglobin. Forensic Sci Int 1987; 33: 1-6.
- Levine B, D’Nicoula J, Kunsman G, Smith M, Stahl C. Methodologic considerations in the interpretation of postmortem carboxyhemoglobin concentrations. Toxicology 1996; 115: 129-34.
- Kunsman G, Presses C, Rodriguez P. Carbon monoxide stability in stored postmortem blood samples. J Anal Toxicol 2000; 24: 572-78.
- Yukawa N, Suzuoka T, Saito T et al. Current CO-oximeters. Forensic Sci Int 1998; 94: 211-15.
- Lee CW, Tam J, Kung LK. Validity of CO-oximetric determination of carboxyhaemoglobin in putrefying blood and body cavity fluid. Forensic Sci Int 2003; 132: 153-56.
- Hampson N. Stability of carboxyhemoglobin in stored and mailed blood samples. Am J Emerg Medicine 2008; 26: 191-95.
- Ghanem A, Rahman R, Shakba O. Stability of carboxyhaemoglobin in blood samples at different periods and temperatures: a forensic and toxicological tool for diagnosis. J Clin Toxicol 2012; 2: 144-47.
- Rosano T. Commentary. Clin Chem 2010; 56: 519-20.
References
- Ranney HM, Sharma V. Structure and function of hemoglobin. In: Beutler E, Lichtman M, eds. Williams Hematology. New York City: McGraw Hill, 2000: 345-53.
- Higgins C. Causes and clinical significance of increased carboxyhemoglobin. www.acutecaretesting.org Oct 2005.
- Higgins C. Methemoglobin. www.acutecaretesting.org Oct 2006.
- Curry S. Hematologic syndromes: hemolysis, methemoglobinemia and sulphhemoglobinemia. In: Brent J, Wallace K, Burkhat K, eds. Critical care toxicology diagnosis and management of the critically poisoned patient. St. Louis: Mosby, 2004.
- Tanggerman A, Bongaerts G, Agbeko R, Semmekrot B, Severijnen R. The origin of hydrogen sulphide in the newborn with sulfhaemoglobin induced cyanosis. J Clin Pathol 2002; 55: 631-33.
- Mahoney J, Vreman H, Stevnson D, Van Kessel A. Measurement of carboxyhemoglobin and total hemoglobin by five specialized spectrohoptometers (CO-oximeters) in comparison with reference methods. Clin Chem 1993; 39: 1693-700.
- Brunelle J, Degtiarov R, Moran R, Race L. Simultaneous measurement of total hemoglobin and its derivatives in blood using CO-oximeters: analytical principles; their application in selecting analytical wavelenghts and reference methods; a comparison of the results of choices made. Scand J Clin Lab Med 1996; 224: 47-69.
- Hitoshu M, Fukita K, Oritani S. Evaluation of postmortem oxymetry with reference to the causes of death. Forensic Sci Int 1997; 87: 201-10.
- Reay D, Insalaco S, Eisele J. Postmortem methemoglobin concentrations and their significance. J Forensic Sci 1984; 29: 1160-63.
- Olsen K, Hillyer M, Kloss J, Geiselhart R, Apple F. Accident or arson: is CO-oximetry reliable for carboxyhemoglobin measurement postmortem? Clin Chem 2010; 56: 515-20.
- Vevelstad M, Morild I. Lethal methemoglobinemia and automobile exhaust inhalation. Forensic Sci Int 2009; 187: 1-5.
- Lewis R, Johnson R, Canfield D. An accurate method for the determination of carbon in postmortem blood using GC-TCD. J Ana Toxicol 2004; 28: 59-62.
- Gauthier S, Grass H, Lory M, Krämer T, Thali M, Bartsch C. Lethal carbon monoxide poisoning in wood pellet storerooms – two cases and a review of the literature. Ann Occup Hyg 2002; 56: 755-63.
- Coburn R, Williams W, Forster RE. Effect of erythrocyte destruction on carbon monoxide production in man. J Clin Invest 1964; 43: 1098-103.
- Ernst A, Zibrak J. Carbon monoxide poisoning. New Eng J Med 1998; 339: 1603-08.
- Lippi G, Rastelli G, Meschi T, Borghi L, Cervellin G. Pathophysiology clinics, diagnosis and treatment of heart involvement in carbon monoxide poisoning. Clin Biochem 2012; 45: 1278-85.
- Widdop B. Analysis of carbon monoxide. Ann Clin Biochem 2002; 39: 378-91.
- Boumba V, Vougiouklakis T. Evaluation of methods used for carboxyhemoglobin analysis in postmortem blood. Int J Toxicol 2005; 24: 275-81.
- Lee CW, Yim LK, Chan DT, Tam JC. Sample pre-treatment for CO-oximetric determination of carboxyhaemoglobin in putrefied blood and cavity fluid. Forensic Sci Int 2002; 126: 162-66.
- Richardson T. Pitfalls in forensic toxicology. Ann Clin Biochem 2000; 37: 20-44.
- Rai V, Minty P. The determination of carboxyhaemoglobin in the presence of sulphaemoglobin. Forensic Sci Int 1987; 33: 1-6.
- Levine B, D’Nicoula J, Kunsman G, Smith M, Stahl C. Methodologic considerations in the interpretation of postmortem carboxyhemoglobin concentrations. Toxicology 1996; 115: 129-34.
- Kunsman G, Presses C, Rodriguez P. Carbon monoxide stability in stored postmortem blood samples. J Anal Toxicol 2000; 24: 572-78.
- Yukawa N, Suzuoka T, Saito T et al. Current CO-oximeters. Forensic Sci Int 1998; 94: 211-15.
- Lee CW, Tam J, Kung LK. Validity of CO-oximetric determination of carboxyhaemoglobin in putrefying blood and body cavity fluid. Forensic Sci Int 2003; 132: 153-56.
- Hampson N. Stability of carboxyhemoglobin in stored and mailed blood samples. Am J Emerg Medicine 2008; 26: 191-95.
- Ghanem A, Rahman R, Shakba O. Stability of carboxyhaemoglobin in blood samples at different periods and temperatures: a forensic and toxicological tool for diagnosis. J Clin Toxicol 2012; 2: 144-47.
- Rosano T. Commentary. Clin Chem 2010; 56: 519-20.
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Acute care testing handbook
Get the acute care testing handbook
Your practical guide to critical parameters in acute care testing.
Download nowRelated webinar
Evolution of blood gas testing Part 1
Presented by Ellis Jacobs, PhD, Assoc. Professor of Pathology, NYU School of Medicine.
Watch the webinar









